The synergistic effect of organic silicone quaternary ammonium salt and 5-fluorouracil on hepatocellular carcinoma in vitro and in vivo
Juan-Xia Wanga,b, Ling-Yi Zhangb, Jun Zhanga,c, Hui Dinga,c, Dong-Min Wanga,c and Zhi-Ping Wanga,c
Hepatocellular carcinoma (HCC) is the third most common type of cancer worldwide, causing over 370 000 deaths per year, with approximately half of them in China.
Chemotherapy is the optimal treatment for patients with advanced HCC, although chemoresistance has become a significant obstacle to successful liver cancer surgery.
In this paper, we have assessed the characteristics of drugs to explore the effects of individual and combined action of organic silicone quaternary ammonium salt (Jieyoushen) and 5-fluorouracil (5-FU). The results of MTT assays showed that single and combined action of Jieyoushen and 5-FU can inhibit the proliferation of liver carcinoma cell lines in a dose-dependent and time-dependent manner, respectively. Electron microscopy and Hoechst 33342 staining showed characteristic apoptotic bodies in apoptotic cells treated with Jieyoushen and 5-FU. Flow cytometry results indicated that the percentage of cells
at G0/G1 phase gradually increased, whereas it gradually decreased during the S phase after treatment.
Taken together, these results suggest that the combination of Jieyoushen with 5-FU exerts a synergistic anticancer effect on HCC growth and that targeted therapeutic strategies may improve HCC sensitivity to chemotherapy. European Journal of Cancer Prevention 23:372–384 §c 2014 Wolters Kluwer Health | Lippincott Williams & Wilkins.
European Journal of Cancer Prevention 2014, 23:372–384
Keywords: apoptosis, 5-fluorouracil, hepatocellular carcinoma, Jieyoushen, synergistic effect
aInstitute of Urology, the Second Hospital of Lanzhou University, bDepartment of Hepatopathy, the Second Hospital of Lanzhou University and cGansu Nephro-Urological Clinical Center, Key Laboratory of Urological Diseases, Lanzhou, China
Correspondence to Zhi-Ping Wang, PhD, Institute of Urology, the Second Hospital of Lanzhou University, No. 82 Cuiyingmen, Lanzhou 730030, Gansu, China
Tel/fax: + 86 931 894 2821; e-mail: erywzp@lzu.edu.cn
Received 12 June 2013 Accepted 25 June 2013
Hepatocellular carcinoma (HCC) is the main form of liver cancer. The incidence of HCC has substantially increased around the world (Chen et al., 2009; Tanizaki et al., 2010; Jemal et al., 2011; Jiao et al., 2012). In China alone, more than 401 000 new patients were diagnosed with HCC and more than 371 000 patients died of this disease in 2008 (Ferlay et al., 2010).
Surgical treatment is considered to be the best choice for HCC (Takayama et al., 2010); however, only a small proportion of patients are candidates for radical resection at the time of diagnosis, owing to the fact that HCC rarely presents with characteristic symptoms in the early stage and over 80% of patients lose their chance for curative hepatectomy when the diagnosis of HCC is confirmed (Jia et al., 2012; Yang et al., 2012). For the management of advanced HCC, systemic chemotherapy with classical cytotoxic agents offers a marginal survival benefit (Thomas et al., 2008; Wang et al., 2012). Compared with local treatments, such as radiation and surgery, chemotherapy is a form of systemic treatment that may reach tumor cells wherever they have spread (Ramalingam and Belani, 2008). At present, more than 100 drugs are being used for chemotherapy, either alone or in combina- tion. However, HCCs are resistant to anticancer drugs
(Asghar and Meyer, 2012; Jia et al., 2012). Traditional systemic chemotherapy has a low curative rate and results in many toxic side effects in liver cancer; hence, it is not widely accepted by clinical practitioners. Recently, the application of new chemotherapeutic drugs and the new combination regimens have been proved to be effective against advanced-stage HCC (Wan et al., 2009; Ca et al., 2012; Xie et al., 2012). Moreover, it is desirable to explore the alternative modalities of combi- nation chemotherapy for advanced HCC.
5-Fluorouracil (5-FU) is one of the optimal drugs for the treatment of HCC. The thymidylate synthase inhibition and the misincorporation of fluoronucleotide into RNA and DNA is generally thought to be the main effective mechanism against cancer (Llovet Josep, 2002; Lopez et al., 2006; Mukai et al., 2006; Xu et al., 2007; Bu et al., 2008). However, the rapid development of acquired resistance to 5-FU has limited its clinical usage (Yoo et al., 2009), and its underlying molecular mechanism remains undefined. To enhance the clinical use of 5-FU, a number of drug combinations have been investigated (Shang et al., 2007; Yang et al., 2008; Morabito et al., 2009). The results of such experimental and clinical studies showed that some drugs can not only be effective against various types of cancer but can also improve the effectiveness of 5-FU
0959-8278 §c 2014 Wolters Kluwer Health | Lippincott Williams & Wilkins DOI: 10.1097/CEJ.0b013e328364f2c8
significantly and reduce toxicity greatly. The combinative mechanism is needed to further identify which one plays the dominant role in the anticancer process.
Jieyoushen is a type of polymeric surfactant, and its main component is a quaternary ammonium salt. Nowadays it is mostly used as a postoperative physical antimicrobial material (Liu et al., 2010). When water-soluble liquid Jieyoushen is sprayed on skin surfaces or mucosal areas, it immediately solidifies and forms an invisible antimicro- bial layer with a dual overlapping structure: the bonded film and the positive-charge film. The bonded film comprises macromolecular agents, securely bonded to the body surface by means of a chemical, which has a long- acting effect on preventing microbial growth. The positive-charge film comprises cationic activators that form a reticulate film with the positive charge of the skin surface or mucosal area. The positive-charge film strongly absorbs pathogenic microorganisms that have negative charge, such as bacteria, fungi, and viruses. If the respiratory enzyme of the pathogenic microorganisms on which they rely for existence is out of action, they will die due to lack of oxygen supply (Li et al., 2011). However, it has not been published in the literature that Jieyoushen is an antitumor agent. The mechanism of tumor metastasis prevention is indeterminate. In our previous research, the anticancer activity of Jieyoushen in vivo was tested in a rat intra-abdominal tumor model, and the result showed that Jieyoushen can prevent the spread of intra-abdominal tumors in rats. Moreover, the incidence rate differed significantly between the treatment and control groups (P < 0.05). Hence, it was concluded that Jieyoushen can be used to prevent tumor implant metastasis when it is used alone or in combination with other chemotherapeutic drugs to decrease the required dose of chemotherapy and minimize toxicity in patients.
In this study, the synergistic effect and mechanisms of action of the combination of Jieyoushen with 5-FU have been investigated in human HCC SMMC7721 cell lines and R15 cell lines of the rat. The synergy, additivity, or antagonism of these agents from growth inhibition, cell cycle distribution, apoptosis, and expression of caspase-3 and caspase-8, Bax, Bcl-2, and survivin proteins were carefully evaluated to understand the combinative action of the two drugs and find the possible mechanism of action. We hope that these results will bring out new therapeutic means for preventing the metastasis of HCC in surgery.
Materials
Jieyoushen was provided by the Nanjing Shenqi Technology Development Co. Ltd (Jiangsu, China). 5-FU (0.25 g/10 ml) was purchased from Tianjin Jinyao Amino Acid Co. Ltd (Tianjin, China). The primary anti-Bax, anti-Bcl-2, anti- caspase-8, anti-caspase-3, anti-survivin, and anti-b-actin
antibodies were obtained from Bioworld Technology Inc. (Louis Park, Minnesota, USA). The annexin V-FITC/ propidium iodide (PI) apoptosis detection kit was purchased from Invitrogen Technology Inc. (Carlsbad, California, USA). Six- to 8-week-old male Wistar rats (Lanzhou University Experimental Animal Center, Lanzhou, China) were used in the in-vivo experiments. A cage, surgical instruments, 10% chloral hydrate (0.35 ml/100 g weight), DMEM high-sugar medium, and fetal calf serum were used.
Cell culture
Human liver carcinoma cell lines (SMMC7721), hepato- cyte lines (LO2), and rat liver carcinoma cell lines (R15) were obtained from American Type Culture Collection (ATCC, Manassas, Virginia, USA). They were cultured in RPMI 1640 medium (Gibco, Grand Island, New York, USA), supplemented with 10% heat-inactivated fetal bovine serum, 50 U/ml of penicillin, and 50 mg/ml of streptomycin, 15 mmol/l (pH 7.2) HEPES, 2 mmol/l L-glutamine, and incubated at 371C in a humidified incubator containing 5% CO2. The treatment groups were divided into different concentrations from 6 to 48 h.
Growth inhibition assay
![]()
![]() Liver carcinoma cells were seeded in 96-well plates at a density of 5 103 cells/well in quintuplicate, and 200 ml Jieyoushen (1/1000, V%), 10 mmol 5-FU, and the combi- nation (1 : 1, V/V) solution were added to each well at a final concentration. Cells were incubated for 0, 6, 12, 24, and 48 h. The serum-free medium group was set as the bottom control, and a positive control group at a density of 5 103 cells/well was also seeded. Meanwhile, hepato- cyte LO2 cells served as the negative control group. After the treatment, 50 mg/10 ml of MTT (Sigma, Santa Clara, California, USA) was mixed into these wells and the cells were further incubated for another 4 h; thereafter, 150 ml of DMSO (Sigma) was added to each well after removing the supernatant. After shaking the plate for 15 min, cell viability was measured by reading the absorbance at a wavelength of 490 nm using a Model 680 Elisa Reader (Bio-Rad, Hercules, California, USA). Survival rates were calculated according to the percentage of positive controls. The experiment was repeated three times.
Liver carcinoma cells were seeded in 96-well plates at a density of 5 103 cells/well in quintuplicate, and 200 ml Jieyoushen (1/1000, V%), 10 mmol 5-FU, and the combi- nation (1 : 1, V/V) solution were added to each well at a final concentration. Cells were incubated for 0, 6, 12, 24, and 48 h. The serum-free medium group was set as the bottom control, and a positive control group at a density of 5 103 cells/well was also seeded. Meanwhile, hepato- cyte LO2 cells served as the negative control group. After the treatment, 50 mg/10 ml of MTT (Sigma, Santa Clara, California, USA) was mixed into these wells and the cells were further incubated for another 4 h; thereafter, 150 ml of DMSO (Sigma) was added to each well after removing the supernatant. After shaking the plate for 15 min, cell viability was measured by reading the absorbance at a wavelength of 490 nm using a Model 680 Elisa Reader (Bio-Rad, Hercules, California, USA). Survival rates were calculated according to the percentage of positive controls. The experiment was repeated three times.
Transmission electron microscopy observation Transmission electron microscopy was performed to detect morphological changes. Briefly, human liver carcinoma cell lines SMMC7721 and rat liver carcinoma cell lines R15 were treated with Jieyoushen (1/1000, V%), 5-FU (10 mmol), and the combination (1 : 1, V/V) for 24 h. After intervention with different concentrations of drugs, cells were digested with 0.25% pancreatin and centri- fuged at 1000 rpm for 5 min, followed by repeated washing with PBS, supernatant removal, and fixing with 2.5% precooled glutaraldehyde overnight at 41C. Ultra- thin sections of copper were prepared, and cells were rinsed once with PBS and then fixed with 1% osmic acid
for 1 h. Samples were then dehydrated by acetone and embedded in epoxide resin. After staining with uranyl acetate and lead citrate, the sections were observed under a transmission electron microscope.
Hoechst staining
![]() Liver carcinoma cells (4 104/well) were cultured in 24-well plates in RPMI 1640, which was supplemented with 10% fetal bovine serum. At the same time, the duplicate culture plates were prepared. Then, human liver carcinoma cell line SMMC7721 and rat liver carcinoma cell line R15 were treated with Jieyoushen (1/1000, V%), 5-FU (10 mmol), and the combination (1 : 1, V/V) for 24 h. After intervention, cells were washed twice with cold PBS (pH 7.2). Then they were fixed with 4% precooled neutral paraformaldehyde overnight at 41C. Thereafter, the cells were washed with PBS and stained with 0.5 ml Hoechst 33342 (Sigma) for 8 min at room temperature, followed
Liver carcinoma cells (4 104/well) were cultured in 24-well plates in RPMI 1640, which was supplemented with 10% fetal bovine serum. At the same time, the duplicate culture plates were prepared. Then, human liver carcinoma cell line SMMC7721 and rat liver carcinoma cell line R15 were treated with Jieyoushen (1/1000, V%), 5-FU (10 mmol), and the combination (1 : 1, V/V) for 24 h. After intervention, cells were washed twice with cold PBS (pH 7.2). Then they were fixed with 4% precooled neutral paraformaldehyde overnight at 41C. Thereafter, the cells were washed with PBS and stained with 0.5 ml Hoechst 33342 (Sigma) for 8 min at room temperature, followed
by another wash with PBS. An excitation wavelength of 350 nm and an emission wavelength of 460 nm were used to observe morphological changes under a fluorescence microscope (Olympus X71; Olympus, Tokyo, Japan).
Flow cytometric analysis of cellular cycle and apoptosis Cells were seeded in 75-cm2 culture flasks. The culture medium was replaced with fresh medium when cells had reached 80% confluency and then cells were exposed to Jieyoushen (1/1000, V%), 5-FU (10 mmol), and the combination (1 : 1, V/V) for 24 h. They were then washed with PBS, fixed in ice-cold 70% ethanol, and stored at
![]() – 201C for further use. Before analysis, cells were washed and suspended at a concentration of 5 106 cells/ml in PBS and incubated with 0.1 mg/ml RNaseA and 40 mg/ml PI at 371C for 20 min. Samples were then analyzed using a FACS Calibur Cell Sorting System (BD Facscalibur; BD Bioscience, Franklin, New Jersey, USA).
– 201C for further use. Before analysis, cells were washed and suspended at a concentration of 5 106 cells/ml in PBS and incubated with 0.1 mg/ml RNaseA and 40 mg/ml PI at 371C for 20 min. Samples were then analyzed using a FACS Calibur Cell Sorting System (BD Facscalibur; BD Bioscience, Franklin, New Jersey, USA).
(A)
 (a)
(a)
(b) (c)
(d) (e)
 (B)
(B)
(a)
(b) (c)
(d) (e)
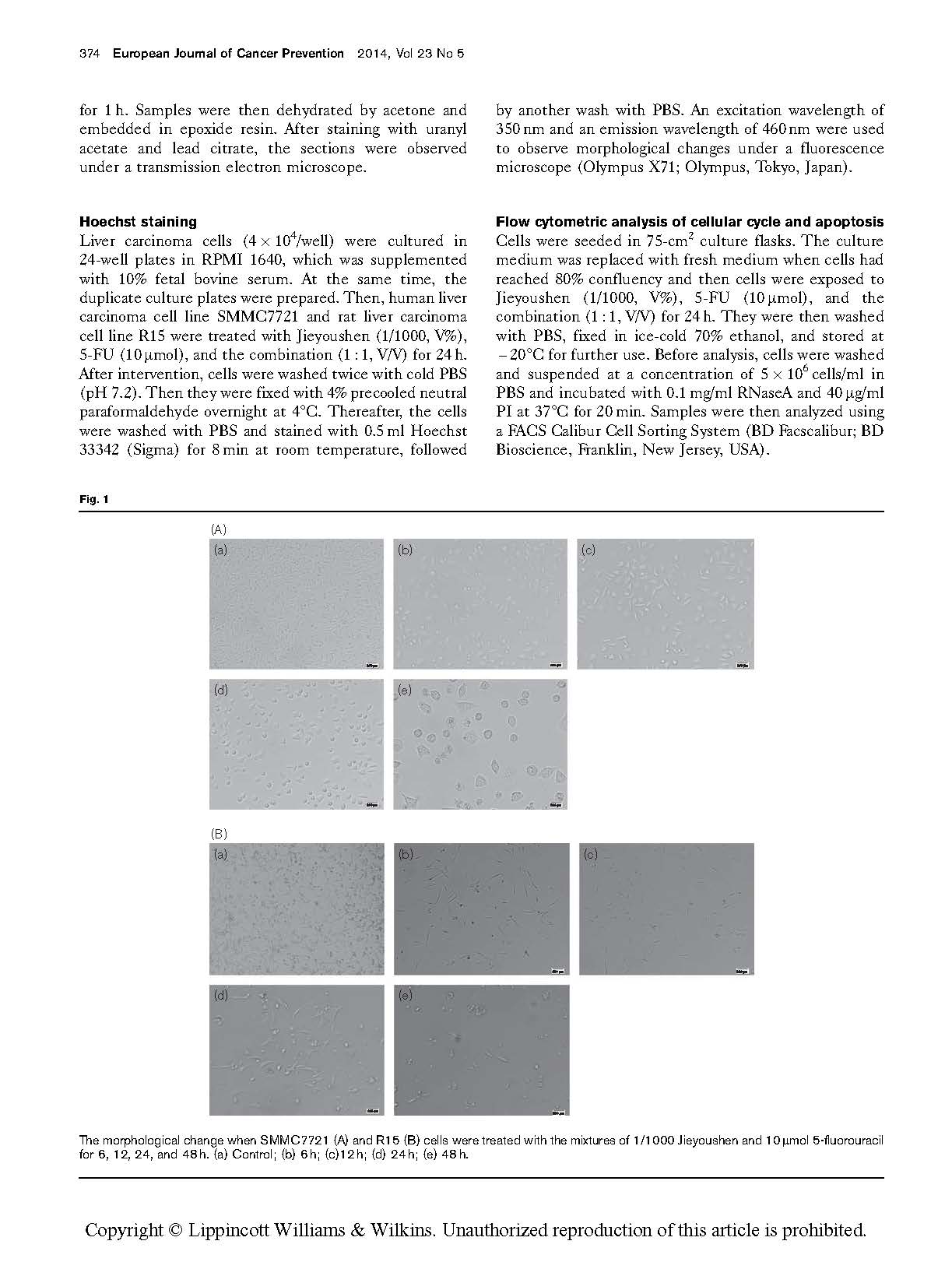
The morphological change when SMMC7721 (A) and R15 (B) cells were treated with the mixtures of 1/1000 Jieyoushen and 10 mmol 5-fluorouracil for 6, 12, 24, and 48 h. (a) Control; (b) 6 h; (c)12 h; (d) 24 h; (e) 48 h.
Western blot analysis of the expression of Bax, Bcl-2, caspase-8, caspase-3, and survivin proteins
Cells were inoculated and fused to 80% confluency and
treated with the same drugs as used in the MTTexperiment. The medium was collected by centrifugation and the cell monolayer was washed with PBS. Protein extraction was carried out using RIPA (Beyotime Biotechnology, Haimen, Jiangsu, China). Cell extracts were collected and centrifuged at 14 000g in a microcentrifuge for 20 min at 41C. The supernatants were collected, and protein concentration was determined using Bradford’s method. Cell extracts were either used immediately or stored at – 801C. They were boiled at 1001C in denaturation buffer for 7 min after taking steps to prevent the degradation of proteins. For SDS-PAGE, 60 mg of protein from each extract was loaded into each well of the gel. A condensation protein electrophoresis gel was run at 80 and 100 V, and proteins were transferred onto polyvinylidene fluoride membranes at 200 mA in an ice bath for 1–2 h. Protein bands were determined using a prestained molecular weight marker (SM0671; Fermentas, Burlington, Ontario, Canada) for reference. Membranes were blocked with TBST buffer (50 mmol/l Tris-HCl, pH 7.4, 150 mmol/l NaCl, 0.1% Tween 20) containing 5% (w/v) fat-free dried milk for 2 h at room temperature and incubated with primary antibody overnight at 41C. Membranes were then washed with TBST and incubated with species-specific HRP- conjugated secondary antibodies for 2 h at room temperature. The membrane underwent additional washing, and the immunoreactive protein was visualized using the chemilu- minescent reagent ECL (Minipore, Darmstadt, Germany) according to the manufacturer’s protocol. Differences in protein expression were examined by densitometry analysis using Image-pro plus 6.0 software (Media Cyberntics Inc, Bethesda, Maryland, USA). The experiments were repeated at least twice to confirm the results.
Animal experiment
![]() Forty male Wistar rats, weighing 150 g, were randomly divided into the control group and the experimental group by a random number table method. R15 cells were collected when they reached 80% or more confluency, and cell vitality was tested using trypan blue staining to ensure that cell vitality was higher than 95%. The cell concentration was diluted to 6 106 /ml with physiological saline. The rats were anesthetized with 10% chloral hydrate, and then fixed on the operating table. We made a 3 cm incision in the middle of the abdomen between the xiphoid process and the pubic symphysis, and then separated the peritoneum using an abdominal tractor. In the treatment group, Jieyoushen (2 ml) was sprayed twice into the abdominal cavity mucous membrane, and then 1 ml cell suspension was absorbed and injected into the enterocoelia. In the control group, 1 ml cell suspension was absorbed and injected into the enterocoelia directly. The abdominal cavity was closed after treatment. The mice were killed with a lethal dose of anesthetic after 5 weeks. We opened the abdominal cavity again beside the old wound. The formation of an implanted metastatic tumor was observed, and the number of rats forming a metastatic tumor was recorded. The tumor growth situation was also recorded by measuring the weight of the tumor. Two tumor tissues were stained with hematoxylin and eosin to observe the pathological features.
Forty male Wistar rats, weighing 150 g, were randomly divided into the control group and the experimental group by a random number table method. R15 cells were collected when they reached 80% or more confluency, and cell vitality was tested using trypan blue staining to ensure that cell vitality was higher than 95%. The cell concentration was diluted to 6 106 /ml with physiological saline. The rats were anesthetized with 10% chloral hydrate, and then fixed on the operating table. We made a 3 cm incision in the middle of the abdomen between the xiphoid process and the pubic symphysis, and then separated the peritoneum using an abdominal tractor. In the treatment group, Jieyoushen (2 ml) was sprayed twice into the abdominal cavity mucous membrane, and then 1 ml cell suspension was absorbed and injected into the enterocoelia. In the control group, 1 ml cell suspension was absorbed and injected into the enterocoelia directly. The abdominal cavity was closed after treatment. The mice were killed with a lethal dose of anesthetic after 5 weeks. We opened the abdominal cavity again beside the old wound. The formation of an implanted metastatic tumor was observed, and the number of rats forming a metastatic tumor was recorded. The tumor growth situation was also recorded by measuring the weight of the tumor. Two tumor tissues were stained with hematoxylin and eosin to observe the pathological features.
Statistical analyses
The results were analyzed using SPSS 15.0 software (SPSS Inc., Chicago, Illinois, USA) and were expressed as mean±SD. One-way analysis of variance and Tukey’s post-hoc tests were used for determining the differences between groups, and a P value of less than 0.05 was considered statistically significant.
1.0
1.0
![]()

![]() 0.8 0.8
0.8 0.8
0.6 0.6
0.4 0.4
0.2 0.2
0.0
 5/10 000 1/1000
5/10 000 1/1000
3/1000 5/1000
0.0
0 10
20 30 40 50
Concentration Concentration (mmol/l)
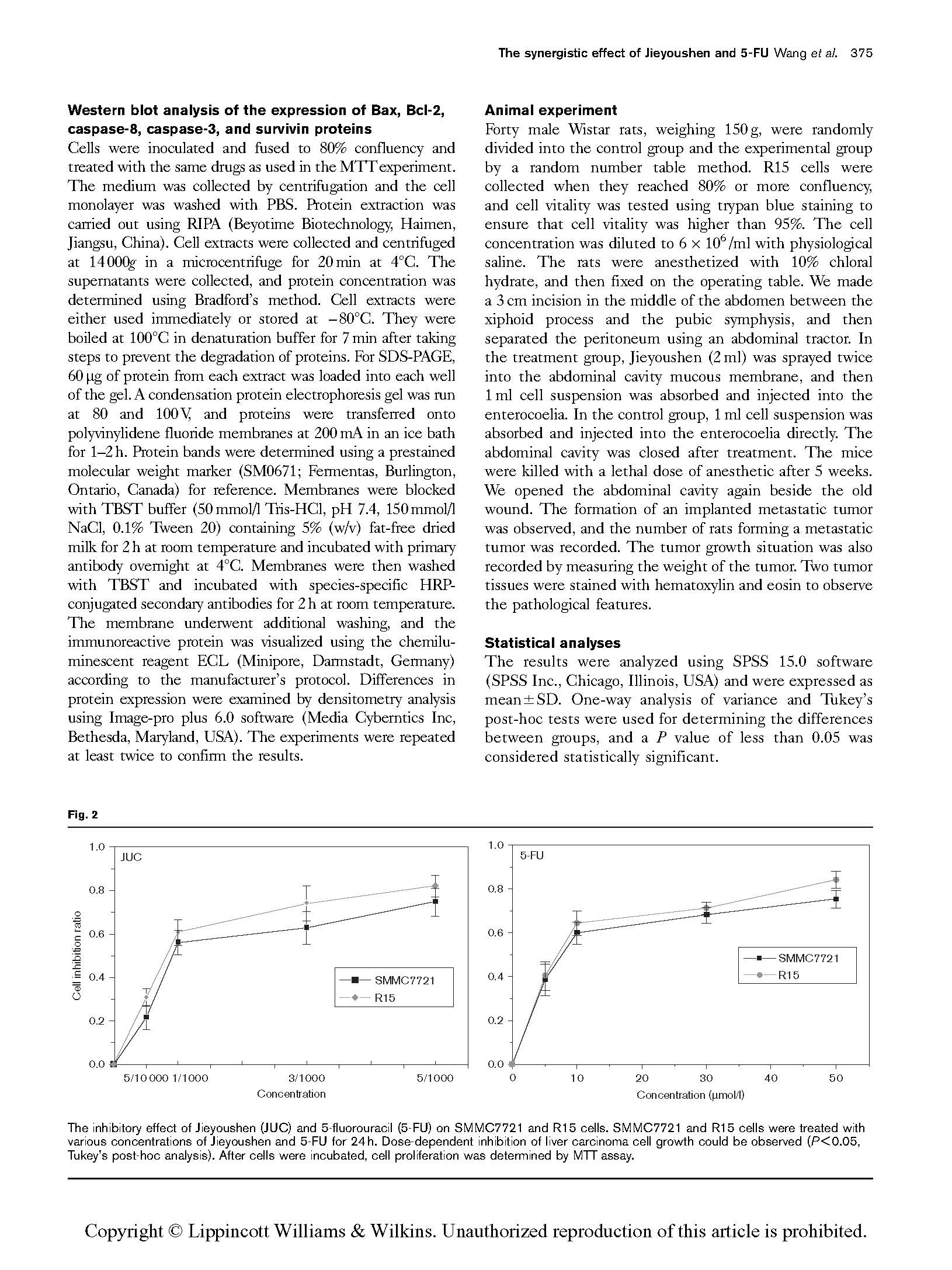
The inhibitory effect of Jieyoushen (JUC) and 5-fluorouracil (5-FU) on SMMC7721 and R15 cells. SMMC7721 and R15 cells were treated with various concentrations of Jieyoushen and 5-FU for 24 h. Dose-dependent inhibition of liver carcinoma cell growth could be observed (P < 0.05, Tukey’s post-hoc analysis). After cells were incubated, cell proliferation was determined by MTT assay.
Morphological observation under light microscopy
It can be seen from Fig. 1 that the cells had changed from the original epithelioid or polygonal shape to round shape, the cell membrane had shrunk, and many nuclei had become pyknotic; the refractive index of the cells had decreased or cytoplasm containing partly multivesicular bodies and dense granules had appeared (Fig. 1).
Cell survival assay
The results of the MTTassay showed that Jieyoushen and 5-FU could inhibit the proliferation of SMMC7721 and R15 cells after 24 h exposure (Fig. 2). Moreover, the observed inhibitory effect was dose dependent. After SMMC7721 and R15 cells had been treated with 5/10 000, 1/1000, 3/1000, and 5/1000 Jieyoushen (dilution rate) for 24 h, the cell inhibition ratios of SMMC7721 were 21.68±5.77, 56.03±5.43, 62.83±7.64, and 74.73±
6.21%, respectively. Similarly, the cell inhibition ratios of R15 were 30.95±4.21, 60.81±5.90, 74.01±7.92, and 82.14±
4.91%, respectively. After SMMC7721 and R15 cells had been treated with 5, 10, 30, and 50 mmol 5-FU for 24 h, the cell inhibition ratios of SMMC7721 were 38.48±7.14, 59.92±5.17, 68.13±3.53, and 75.27±4.26%, respectively. Similarly, the cell inhibition ratios of R15 were 40.18±6.34, 64.33±5.47, 71.24±2.93, and 84.12±3.91%, respectively. Considering the comprehensive contrast effect, 1/1000 Jieyoushen and 10 mmol 5-FU were chosen to investigate the combinative effect of Jieyoushen and 5-FU in inhibiting the growth of HCC cell lines.
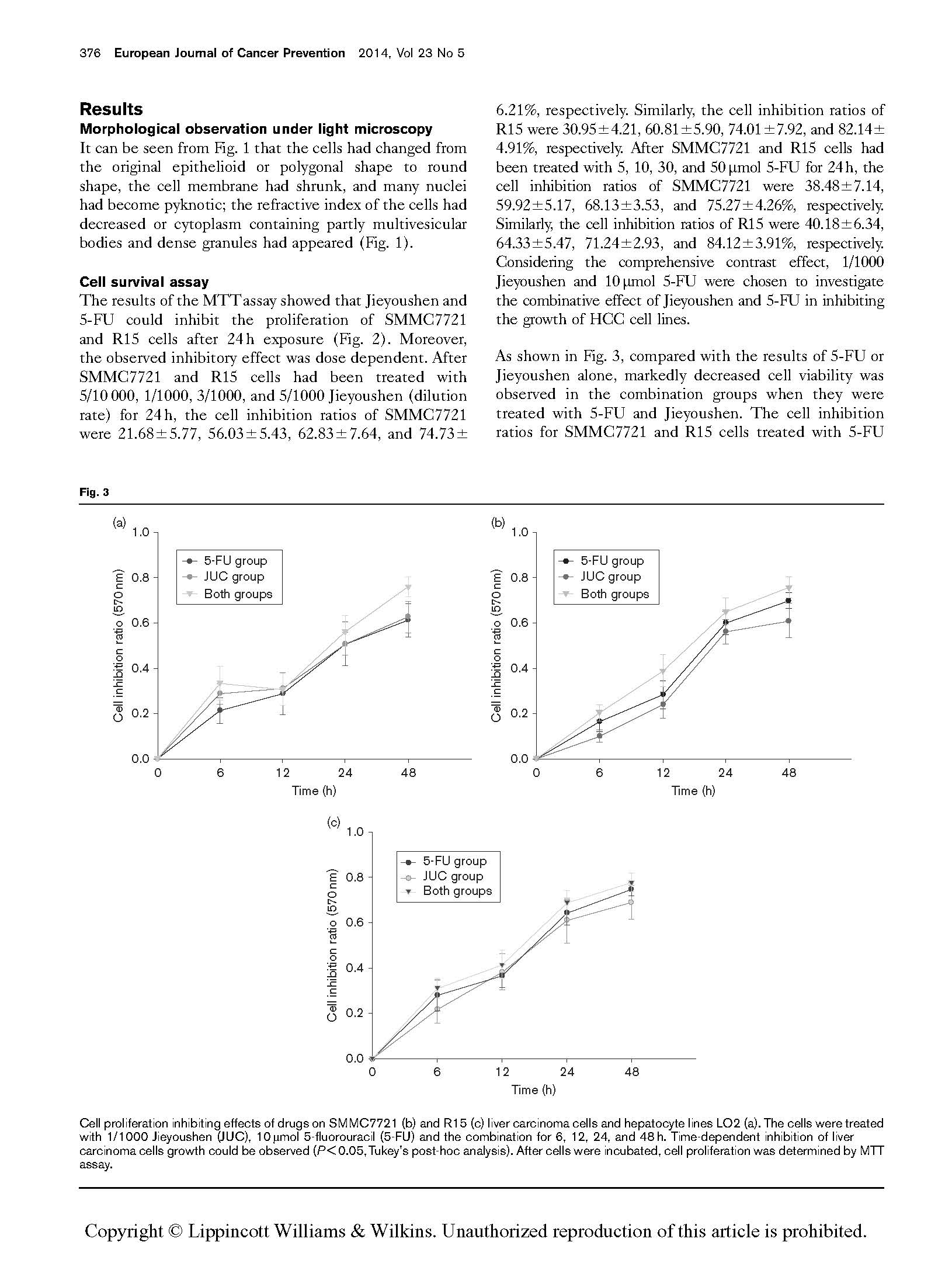
As shown in Fig. 3, compared with the results of 5-FU or Jieyoushen alone, markedly decreased cell viability was observed in the combination groups when they were treated with 5-FU and Jieyoushen. The cell inhibition ratios for SMMC7721 and R15 cells treated with 5-FU
(a) 1.0

 (b) 1.0
(b) 1.0
![]()
![]() 0.8 0.8
0.8 0.8
0.6 0.6
0.4 0.4
0.2 0.2
0.0
0 6 12 24 48
Time (h)
 (c) 1.0
(c) 1.0
0.0
0 6 12 24 48
Time (h)
![]() 0.8
0.8
0.6
0.4
0.2
0.0
0 6 12 24 48
Time (h)
Cell proliferation inhibiting effects of drugs on SMMC7721 (b) and R15 (c) liver carcinoma cells and hepatocyte lines LO2 (a). The cells were treated with 1/1000 Jieyoushen (JUC), 10 mmol 5-fluorouracil (5-FU) and the combination for 6, 12, 24, and 48 h. Time-dependent inhibition of liver carcinoma cells growth could be observed (P < 0.05, Tukey’s post-hoc analysis). After cells were incubated, cell proliferation was determined by MTT assay.
 |
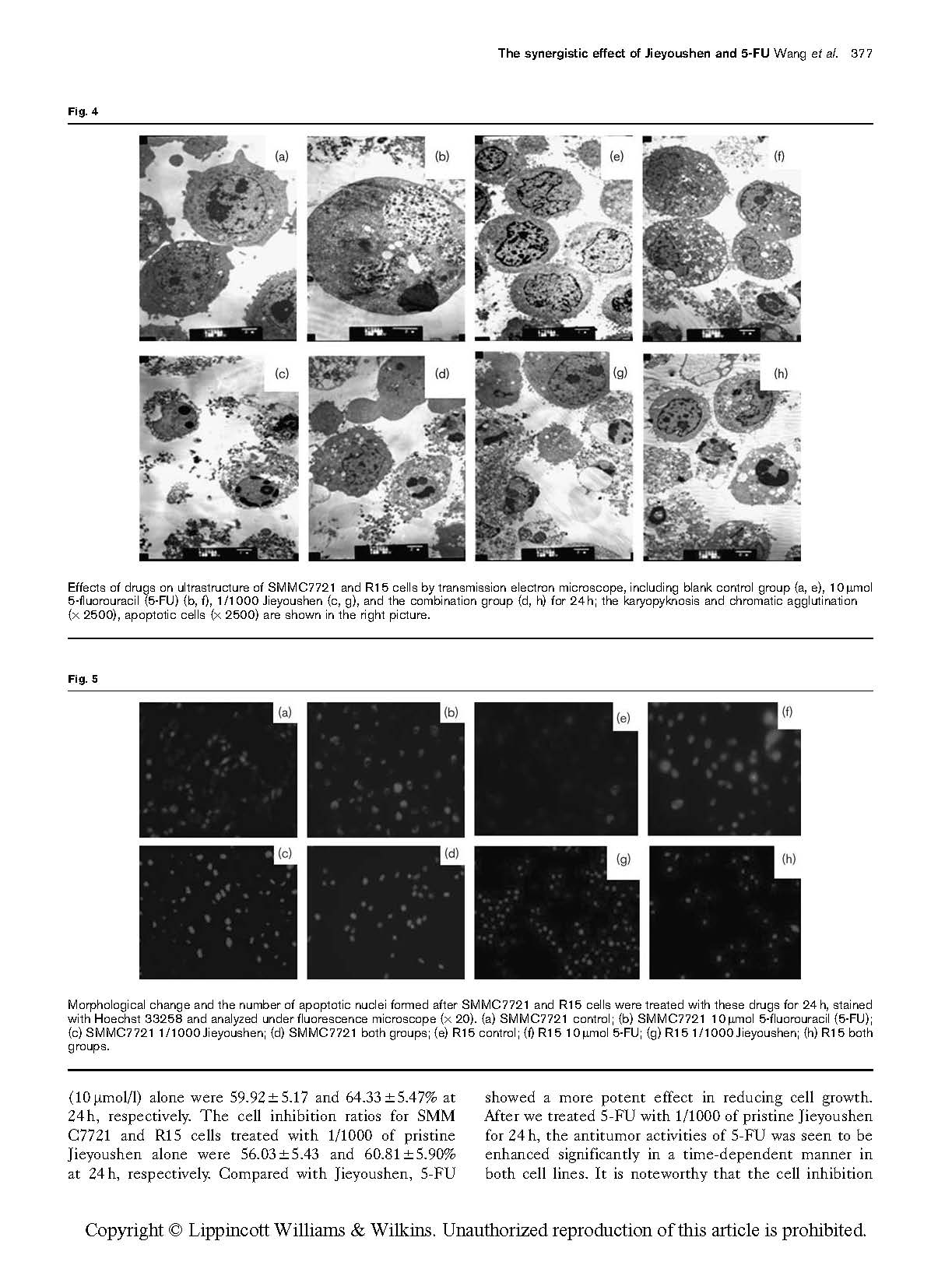
Effects of drugs on ultrastructure of SMMC7721 and R15 cells by transmission electron microscope, including blank control group (a, e), 10 mmol 5-fluorouracil (5-FU) (b, f), 1/1000 Jieyoushen (c, g), and the combination group (d, h) for 24 h; the karyopyknosis and chromatic agglutination (× 2500), apoptotic cells (× 2500) are shown in the right picture.
 |
![]() Morphological change and the number of apoptotic nuclei formed after SMMC7721 and R15 cells were treated with these drugs for 24 h, stained with Hoechst 33258 and analyzed under fluorescence microscope ( 20). (a) SMMC7721 control; (b) SMMC7721 10 mmol 5-fluorouracil (5-FU);
Morphological change and the number of apoptotic nuclei formed after SMMC7721 and R15 cells were treated with these drugs for 24 h, stained with Hoechst 33258 and analyzed under fluorescence microscope ( 20). (a) SMMC7721 control; (b) SMMC7721 10 mmol 5-fluorouracil (5-FU);
(c) SMMC7721 1/1000 Jieyoushen; (d) SMMC7721 both groups; (e) R15 control; (f) R15 10 mmol 5-FU; (g) R15 1/1000 Jieyoushen; (h) R15 both groups.
(10 mmol/l) alone were 59.92±5.17 and 64.33±5.47% at 24 h, respectively. The cell inhibition ratios for SMM C7721 and R15 cells treated with 1/1000 of pristine Jieyoushen alone were 56.03±5.43 and 60.81±5.90% at 24 h, respectively. Compared with Jieyoushen, 5-FU
showed a more potent effect in reducing cell growth. After we treated 5-FU with 1/1000 of pristine Jieyoushen for 24 h, the antitumor activities of 5-FU was seen to be enhanced significantly in a time-dependent manner in both cell lines. It is noteworthy that the cell inhibition
(a)
2340 CV G21 S0
 1950
1950
900
750
CV G21 S0
![]() 1560
1560
1170
780
390
0
540
450
![]() 360
360
270
180
90
Control
0 32 64 96 128 160 192 224 256
CV G21 S0
JUC
600
450
300
150
0
480
400
320
240
160
80
5-FU
0 32 64 96 128 160 192 224 256
CV G21 S0
Both
(b)
0
240
0 32 64 96 128 160 192 224 256
DNA content
CV G21 S0
0
600
0 32 64 96 128 160 192 224 256
DNA content
 CV G21 S0
CV G21 S0
![]() 5-FU
5-FU
360
300
![]() 240
240
180
120
60
0
0 32 64 96 128 160 192 224 256
CV G21 S0
JUC
0 32 64 96 128 160 192 224 256
DNA content
180
150
120
90
60
30
0
0 32 64 96 128 160 192 224 256
CV G21 S0
Both
0 32 64 96 128 160 192 224 256
DNA content
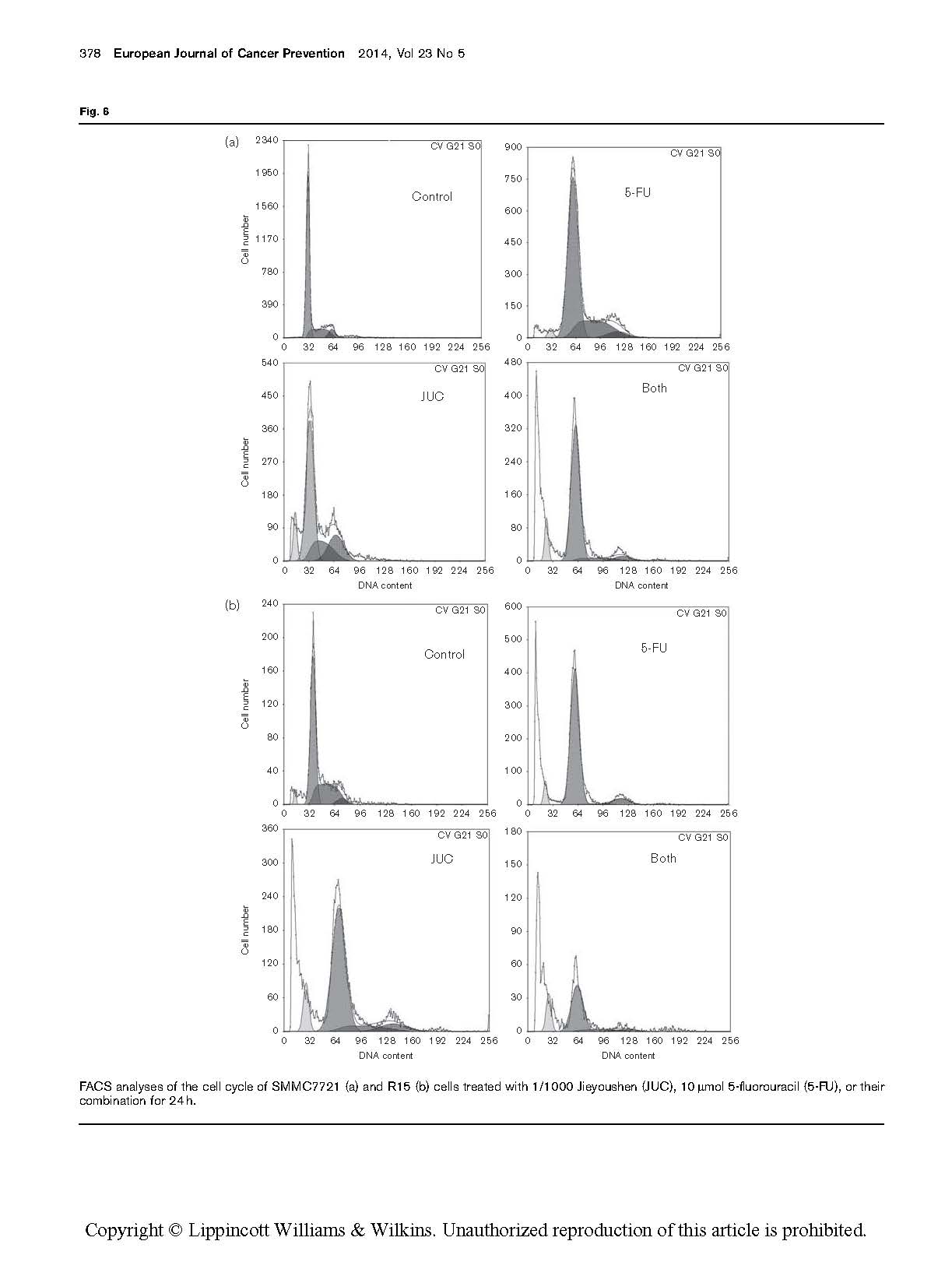
FACS analyses of the cell cycle of SMMC7721 (a) and R15 (b) cells treated with 1/1000 Jieyoushen (JUC), 10 mmol 5-fluorouracil (5-FU), or their combination for 24 h.
ratios for SMMC7721 and R15 cells treated with 1/1000 of pristine Jieyoushen plus 10 mmol/l 5-FU increased to 65.05±5.87 and 68.67±5.29%, respectively. These data indicate that the combination of Jieyoushen and 5-FU synergistically inhibits cell growth in HCC cell lines.
Transmission electron microscopy for ultrastructural changes and analysis of apoptosis
It can be seen from Fig. 4 that SMMC7721 and R15 cells
showed increased heterochromatin within the nucleus, as well as chromatin margination, chromatin clump shrinkage within the cytoplasm, incomplete cell membranes, and increasing numbers of apoptotic cells, necrotic cells, or autophagic bubbles within the cytoplasm. Besides the above ultrastructural changes, R15 cells showed a large number of autophagic vesicles and apoptotic cells, and more cells dissolved and were necrotic. This evidence suggests that the joint action of 5-FU and Jieyoushen could inhibit the proliferation of SMMC7721 and R15 cells.
Hoechst staining
Hoechst 33342 staining was used to observe the morphology of apoptotic cells. The results showed that the control group exhibited regular nuclear morphology, uniform staining, and light blue color. After the joint action for 24 h, compared with the control group, cell morphology reduced and nuclear condensation or fragmentation block formed characteristic apoptotic bodies (Fig. 5).
Flow cytometric detection of cell cycle and apoptosis
Cell cycle analysis
The cell cycle results showed that the two cell lines underwent the same changes in cell cycle, which are described below.
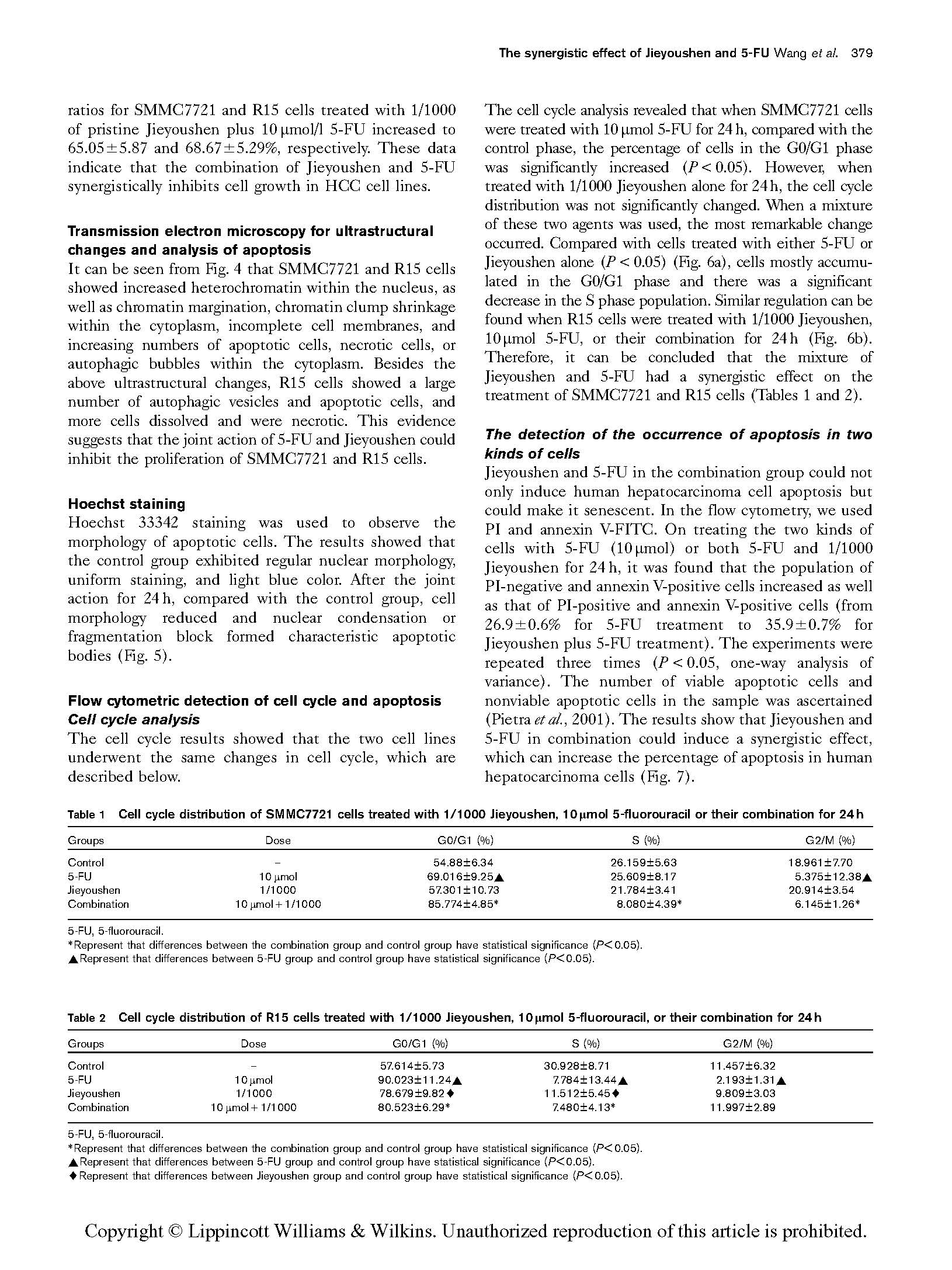
The cell cycle analysis revealed that when SMMC7721 cells were treated with 10 mmol 5-FU for 24 h, compared with the control phase, the percentage of cells in the G0/G1 phase was significantly increased (P < 0.05). However, when treated with 1/1000 Jieyoushen alone for 24 h, the cell cycle distribution was not significantly changed. When a mixture of these two agents was used, the most remarkable change occurred. Compared with cells treated with either 5-FU or Jieyoushen alone (P < 0.05) (Fig. 6a), cells mostly accumu- lated in the G0/G1 phase and there was a significant decrease in the S phase population. Similar regulation can be found when R15 cells were treated with 1/1000 Jieyoushen, 10 mmol 5-FU, or their combination for 24 h (Fig. 6b). Therefore, it can be concluded that the mixture of Jieyoushen and 5-FU had a synergistic effect on the treatment of SMMC7721 and R15 cells (Tables 1 and 2).
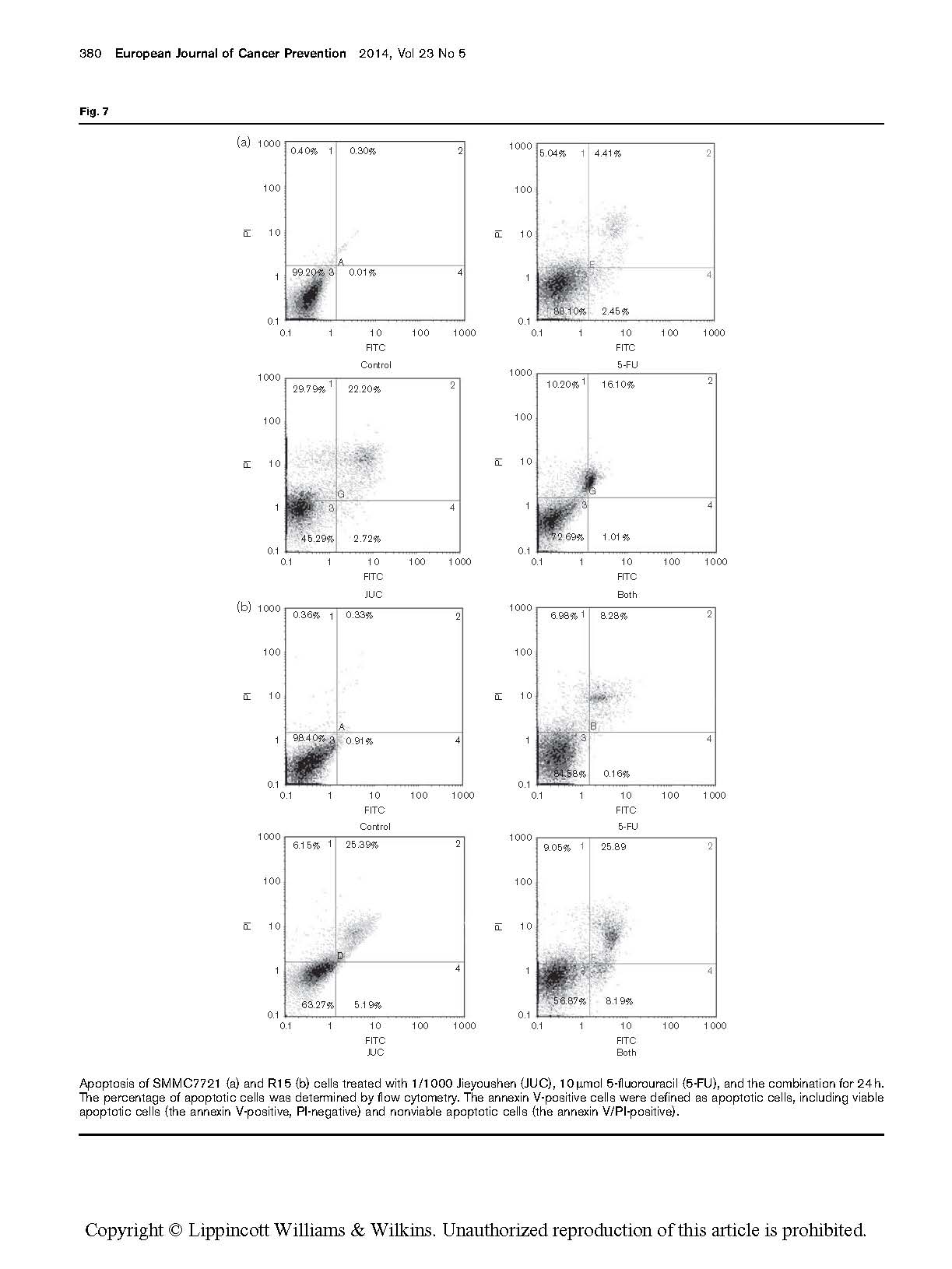
The detection of the occurrence of apoptosis in two kinds of cells
Jieyoushen and 5-FU in the combination group could not
only induce human hepatocarcinoma cell apoptosis but could make it senescent. In the flow cytometry, we used PI and annexin V-FITC. On treating the two kinds of cells with 5-FU (10 mmol) or both 5-FU and 1/1000 Jieyoushen for 24 h, it was found that the population of PI-negative and annexin V-positive cells increased as well as that of PI-positive and annexin V-positive cells (from 26.9±0.6% for 5-FU treatment to 35.9±0.7% for Jieyoushen plus 5-FU treatment). The experiments were repeated three times (P < 0.05, one-way analysis of variance). The number of viable apoptotic cells and nonviable apoptotic cells in the sample was ascertained (Pietra et al., 2001). The results show that Jieyoushen and 5-FU in combination could induce a synergistic effect, which can increase the percentage of apoptosis in human hepatocarcinoma cells (Fig. 7).
Table 1 Cell cycle distribution of SMMC7721 cells treated with 1/1000 Jieyoushen, 10 lmol 5-fluorouracil or their combination for 24 h
Groups | Dose | G0/G1 (%) | S (%) | G2/M (%) |
Control | – | 54.88±6.34 | 26.159±5.63 | 18.961±7.70 |
5-FU | 10 mmol | 69.016±9.25~ | 25.609±8.17 | 5.375±12.38~ |
Jieyoushen | 1/1000 | 57.301±10.73 | 21.784±3.41 | 20.914±3.54 |
Combination | 10 mmol + 1/1000 | 85.774±4.85* | 8.080±4.39* | 6.145±1.26* |
5-FU, 5-fluorouracil.
*Represent that differences between the combination group and control group have statistical significance (P < 0.05).
~Represent that differences between 5-FU group and control group have statistical significance (P < 0.05).
Table 2 Cell cycle distribution of R15 cells treated with 1/1000 Jieyoushen, 10 lmol 5-fluorouracil, or their combination for 24 h
Groups | Dose G0/G1 (%) | S (%) | G2/M (%) |
Control | – 57.614±5.73 | 30.928±8.71 | 11.457±6.32 |
5-FU | 10 mmol 90.023±11.24~ | 7.784±13.44~ | 2.193±1.31~ |
Jieyoushen | 1/1000 78.679±9.82~ | 11.512±5.45~ | 9.809±3.03 |
Combination | 10 mmol + 1/1000 80.523±6.29* | 7.480±4.13* | 11.997±2.89 |
5-FU, 5-fluorouracil.
*Represent that differences between the combination group and control group have statistical significance (P < 0.05).
~Represent that differences between 5-FU group and control group have statistical significance (P < 0.05).
~Represent that differences between Jieyoushen group and control group have statistical significance (P < 0.05).
(a) 1000
0.40% 1
0.30%
2 1000 5.04% 1 4.41% 2
100
![]() 10
10
A
1 99.20% 3 0.01%
100
![]() 10
10
E
4 1 3 4
0.1
0.1
88.10% 2.45%
0.1 1
10 100 1000
FITC
Control
0.1 1
1000
10 100 1000
FITC
5-FU
1000
100
![]() 10
10
29.79% 1 22.20%
2
100
![]() 10
10
10.20% 1
16.10% 2
G G
1 3 4 1 3 4
0.1
45.29% 2.72%
0.1
72.69% 1.01%
 0.1 1
0.1 1
10 100 1000 0.1 1
FITC
JUC
10 100 1000
FITC
Both
(b) 1000
100
![]() 10
10
0.36% 1
0.33%
1000
 2
2
100
![]() 10
10
6.98% 1
8.28% 2
1 98.40% 3
A
0.91%
B
4 1 3 4
0.1
0.1
84.58% 0.16%
0.1 1
10 100 1000 0.1 1
FITC
Control
10 100 1000
FITC
5-FU
1000
100
![]() 10
10
1
6.15% 1
3
25.39%
D
2 1000
100
![]() 10
10
4 1
9.05%
1 25.89 2
F
3 4
0.1
63.27% 5.19%
0.1
56.87% 8.19%
0.1 1
10 100 1000 0.1 1
FITC JUC
10 100 1000
FITC
Both
Apoptosis of SMMC7721 (a) and R15 (b) cells treated with 1/1000 Jieyoushen (JUC), 10 mmol 5-fluorouracil (5-FU), and the combination for 24 h. The percentage of apoptotic cells was determined by flow cytometry. The annexin V-positive cells were defined as apoptotic cells, including viable apoptotic cells (the annexin V-positive, PI-negative) and nonviable apoptotic cells (the annexin V/PI-positive).
SMME7721
Survivin Bax
Bcl-2
R15
SMME7721
Survivin Bax
Bcl-2
R15
 Control 5-FU JUC Both
Control 5-FU JUC Both
Caspases-8 Caspases-3
b-Actin
Caspases-8 Caspases-3


 b-Actin
b-Actin
Control 5-FU JUC Both 6h 12 h 24h 48 h 6 h 12 h 24 h 48 h
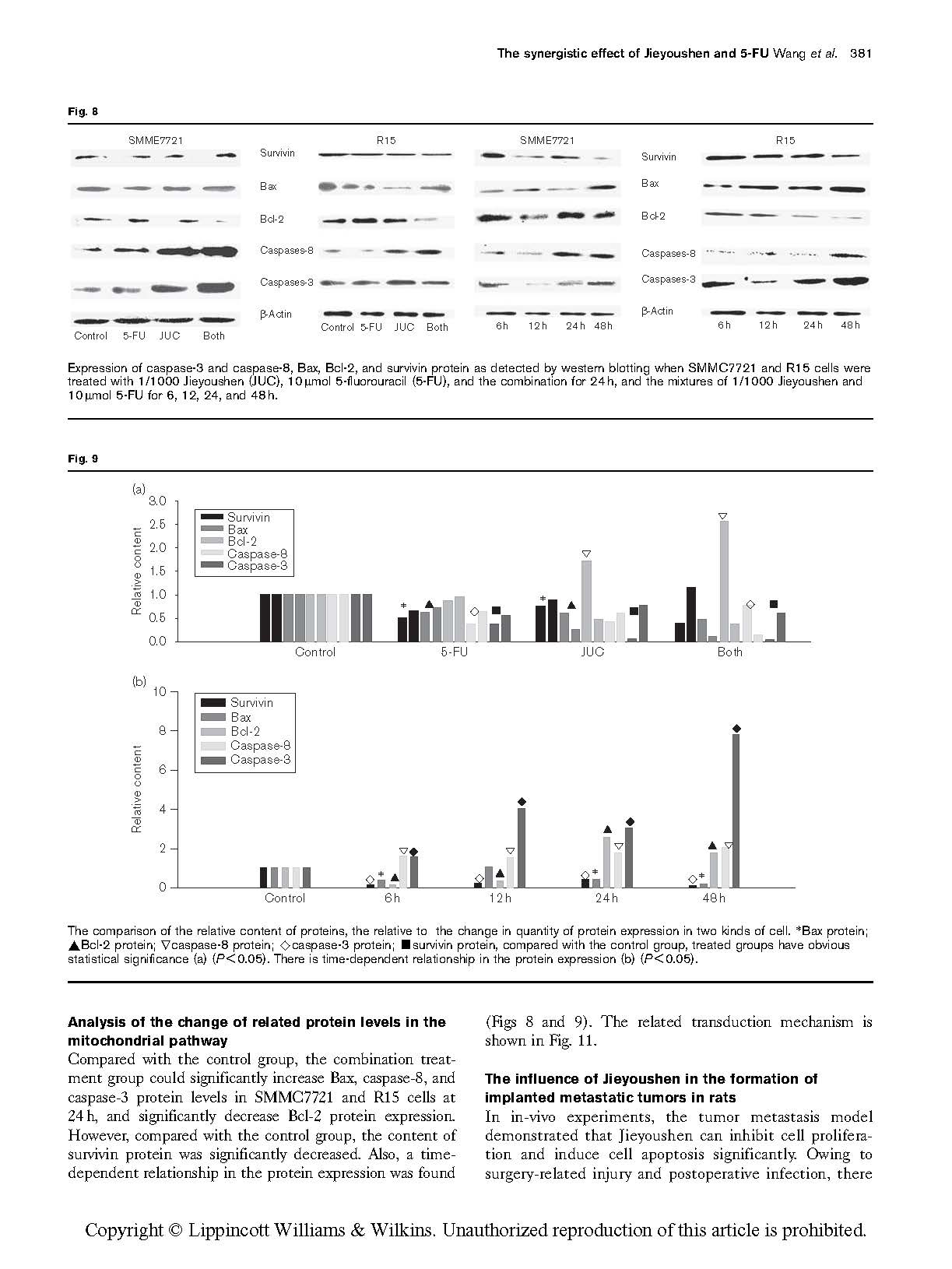
Expression of caspase-3 and caspase-8, Bax, Bcl-2, and survivin protein as detected by western blotting when SMMC7721 and R15 cells were treated with 1/1000 Jieyoushen (JUC), 10 mmol 5-fluorouracil (5-FU), and the combination for 24 h, and the mixtures of 1/1000 Jieyoushen and 10 mmol 5-FU for 6, 12, 24, and 48 h.
![]() Fig. 9
Fig. 9
(a)
3.0
![]() 2.5
2.5
2.0
1.5
1.0
0.5
0.0
(b) 10
![]() 8
8
6
4
2
 Control 5-FU
Control 5-FU
JUC Both
 0
0
Control 6 h
12 h
24 h
48 h
The comparison of the relative content of proteins, the relative to the change in quantity of protein expression in two kinds of cell. *Bax protein;
![]() ~Bcl-2 protein; caspase-8 protein; ^caspase-3 protein; ’survivin protein, compared with the control group, treated groups have obvious statistical significance (a) (P < 0.05). There is time-dependent relationship in the protein expression (b) (P < 0.05).
~Bcl-2 protein; caspase-8 protein; ^caspase-3 protein; ’survivin protein, compared with the control group, treated groups have obvious statistical significance (a) (P < 0.05). There is time-dependent relationship in the protein expression (b) (P < 0.05).
Analysis of the change of related protein levels in the mitochondrial pathway
Compared with the control group, the combination treat-
ment group could significantly increase Bax, caspase-8, and caspase-3 protein levels in SMMC7721 and R15 cells at 24 h, and significantly decrease Bcl-2 protein expression. However, compared with the control group, the content of survivin protein was significantly decreased. Also, a time- dependent relationship in the protein expression was found
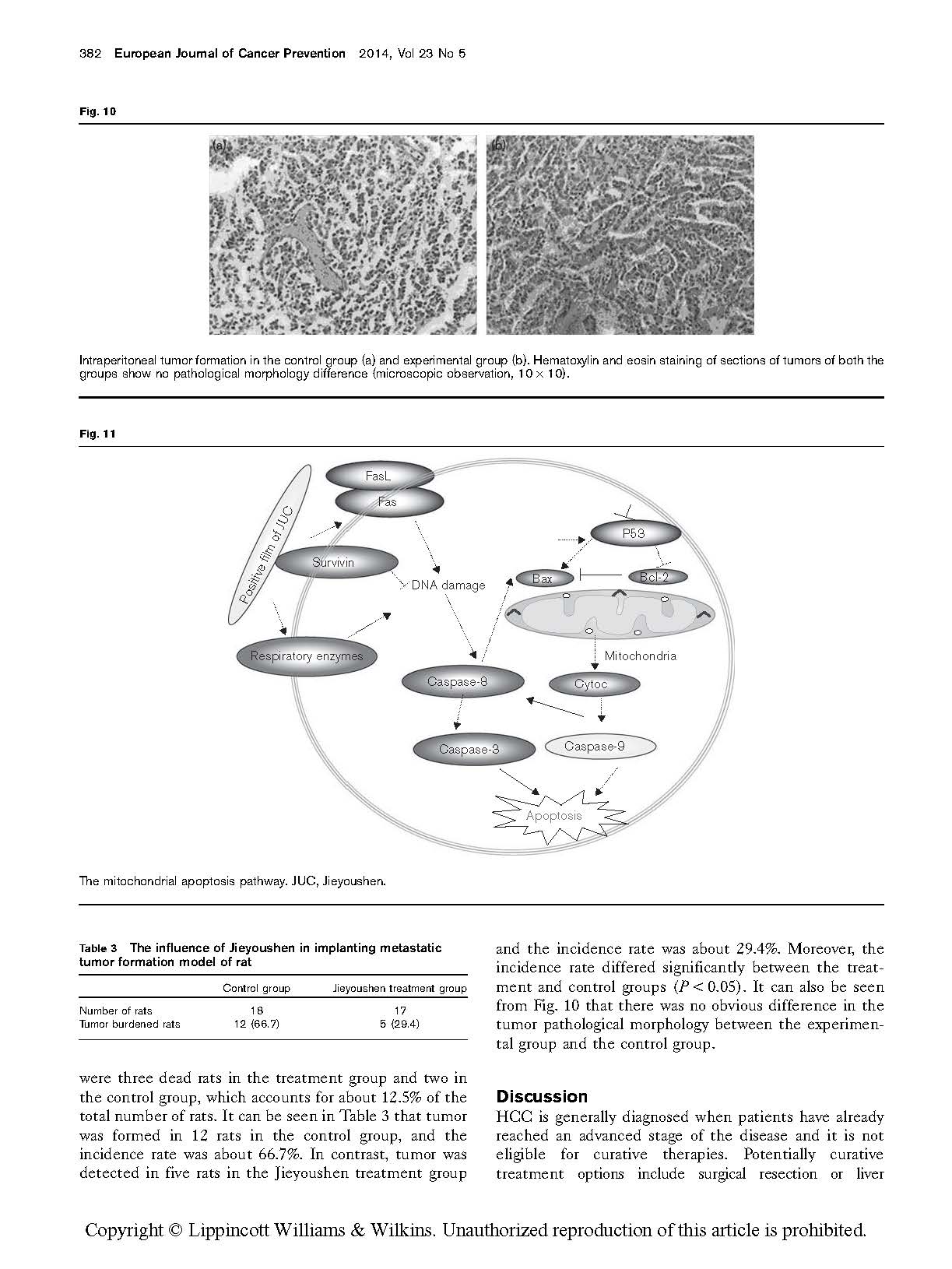
(Figs 8 and 9). The related transduction mechanism is shown in Fig. 11.
The influence of Jieyoushen in the formation of implanted metastatic tumors in rats
In in-vivo experiments, the tumor metastasis model
demonstrated that Jieyoushen can inhibit cell prolifera- tion and induce cell apoptosis significantly. Owing to surgery-related injury and postoperative infection, there
 |
Intraperitoneal tumor formation in the control group (a) and experimental group (b). Hematoxylin and eosin staining of sections of tumors of both the groups show no pathological morphology difference (microscopic observation, 10 × 10).
 The mitochondrial apoptosis pathway. JUC, Jieyoushen.
The mitochondrial apoptosis pathway. JUC, Jieyoushen.
Table 3 The influence of Jieyoushen in implanting metastatic tumor formation model of rat
![]()
![]() Control group Jieyoushen treatment group
Control group Jieyoushen treatment group
Number of rats 18 17
![]() Tumor burdened rats 12 (66.7) 5 (29.4)
Tumor burdened rats 12 (66.7) 5 (29.4)
were three dead rats in the treatment group and two in the control group, which accounts for about 12.5% of the total number of rats. It can be seen in Table 3 that tumor was formed in 12 rats in the control group, and the incidence rate was about 66.7%. In contrast, tumor was detected in five rats in the Jieyoushen treatment group
and the incidence rate was about 29.4%. Moreover, the incidence rate differed significantly between the treat- ment and control groups (P < 0.05). It can also be seen from Fig. 10 that there was no obvious difference in the tumor pathological morphology between the experimen- tal group and the control group.
HCC is generally diagnosed when patients have already reached an advanced stage of the disease and it is not eligible for curative therapies. Potentially curative treatment options include surgical resection or liver
transplantation, which can offer patients adequate liver function and tumor treatment (Muller, 2006). Implantation metastasis is difficult to avoid during operation, which leads to the relapse of liver cancer. In addition, in many cases, implantation metastases are a predominant cause of morbidity and mortality. Because of the high incidence of implantation metastases, regional strategies directed toward the liver have been developed in an attempt to improve patient survival. The individuals not eligible for curative therapy are generally treated with local ablative methods such as radiofrequency ablation or alcohol ablation, and systemic chemotherapy can be offered either alone or in combination to selected groups of patients. However, HCC is not sensitive to chemotherapy, and the development of side effects and drug resistance is the biggest challenge for chemotherapy. Multiple factors are implicated in increasing resistance to chemotherapeutic agents, including reduction of intracellular drug accumulation, DNA damage repair by the modulation of proliferative or antiapoptotic proteins, etc. (Siddik, 2003). These causes eventually lead to an unsatisfactory clinical therapeutic effect. Therefore, more and more research is being carried out to solve this difficult problem. According to the characteristics of each drug, the researchers have adopted the synergistic effect of several drugs to treat diseases. A combination of drugs is recommended to optimize the treatment schedules, in order to achieve the best form of therapy and/or minimize side effects (Eichhorn et al., 2004; Johnston et al., 2007).
In animal models, Jieyoushen has been proved to be very successful in inhibiting tumor development. Therefore, Jieyoushen can be a useful adjunct for improving the effectiveness of chemotherapeutic agents in the treatment of liver cancer. 5-FU and its derivatives, as a classic chemotherapy drug, can cause cell injury by inhibiting thymidylate synthesis. It has become a mainstay of treatment for advanced-stage HCC. However, the evolution of HCC chemotherapy was delayed by the resistance to 5-FU and side effects (Yau et al., 2008; Yang et al., 2012). The required dose must be decreased and the host toxicity must be minimized to improve the effects of 5-FU. In our present study, compared with either agent alone, the combination of Jieyoushen with 5-FU showed a better inhibitory effect. The possible mechanism is that Jieyoushen had been sprayed on the surface of tissues, which formed an invisible protective film, which can prevent the adhesion of the tumor cell to the treated tissue surface. The mechanism is different from that of other general antitumor drugs. Jieyoushen not only has a chemical–drug-directed antitumor effect, but can also form a layer of protective film on the tissue surface, which can prevent cancer cells from growing in organization.
In principle, survival signals are ideal targets for anti- cancer therapeutic strategies because blocking these signals leads to the death of cells that are dependent on them. Our experiment showed that the combination treatment of Jieyoushen with 5-FU significantly inhibited
HCC growth. The apoptosis-related protein in the mito- chondrial pathway that may cause the cascade effect of additional functional proteins may be involved in this inhibition. In this study, we observed that the induction of apoptosis by the combination of Jieyoushen and 5-FU seems to be mediated through the modulation of protein levels of the Bcl-2 family, such as Bcl-2 and Bax. Moreover, p53 plays important roles in Jieyoushen and 5-FU-induced apoptosis in HCC cells.
It is well known that the interplay between members of the Bcl-2 protein family can regulate the mitochondrial (or intrinsic) apoptosis pathway (Lei et al., 2012). Following apoptotic stimuli, cytochrome c is released into the cytosol from the mitochondria as a result of mitochondrial outer membrane permeabilization, which is followed by the formation of apoptotic bodies and caspase activation (Danial and Korsmeyer, 2004). The key, essentially irreversible, step in the commitment to apoptotic death is the permeabiliza- tion of the mitochondrial outer membrane. Bcl-2 is an upstream effector molecule in the apoptotic pathway and has been identified as a potent suppressor of apoptosis (Hockenbery et al., 1993).
We found that the combination of Jieyoushen and 5-FU significantly downregulated Bcl-2 protein and upregulated levels of Bax protein in SMMC7721 and R15 cells. These suggest an involvement of an intrinsic apoptotic pathway by which alantolactone induces apoptosis in SMMC7721 and R15 cells. However, western blot analysis revealed that Jieyoushen, in combination with 5-FU, led to enhanced processing of initiator caspase-8 in SMMC7721 and R15 cells, and these data suggest a possibility of a cross-talk between the two pathways, as further activated caspase-8 can activate caspase-3. Therefore, effective inhibition of caspase-3 and caspase-9 could be critical in providing a targeted pathway for cancer prevention and treatment. Finally, the caspase-3 protein executed the instruction of apoptosis, leading to cell death. We observed that Jieyoushen, in combination with 5-FU, led to a dose-dependent decrease in the protein level in SMMC7721 and R15 cells, which may downregulate the expression of antiapoptotic target genes such as Bcl-2, from which we can conclude that these may favor the apoptosis of SMMC7721 and R15 cells. p53 is a transcription factor that regulates cell cycle progression and DNA repair, and we infer that this may favor apoptosis of SMMC7721 and R15 cells. This multitasking is important for the suppression of tumor formation, as well as for mediating the cellular responses to many standard DNA damage-inducing cancer therapies (Vazquez et al., 2008). When the combination of Jieyoushen and 5-FU was used, the DNA damage and cell cycle change were first caused by an internal mechanism.
Our result indicates that the combination of Jieyoushen and 5-FU significantly increased the expression of p53 in SMMC7721 and R15 cells, which may lead to cell cycle arrest and upregulation of Bax expression (Rocha and Perkins, 2005). These show that p53 is an independent factor in the
combination of Jieyoushen with 5-FU that induced apoptosis. Meanwhile, the nano-positively charged layer damaged the cell membrane. Further, the drugs damaged the cell directly, and then caspase-8 protein was activated and influenced the proteins of the mitochondrial membrane, such as Bax, Bcl-2, and so on. These led to the apoptosis of the classic mitochondrial pathway. This mechanism is shown in Fig. 11.
In summary, the combination of Jieyoushen with 5-FU could inhibit the proliferation of liver carcinoma cell lines and prompt cell apoptosis, which originated from the shielding effect of the positive film. This physical film can render cell respiration enzymes anaerobic and induce cell death (He et al., 2012). Finally, the cell membrane signal ended. The ability of the combination of Jieyoushen with 5-FU to inhibit cell proliferation and induce apoptosis is caused by a multipronged cascade of events. In future studies, the complex coupling reaction that occurred between the positive membrane and the membrane sur- face will be further discussed. As both agents have been widely used in clinical practice, intraoperative cases of metastasis are necessary to identify the curative effect of combined treatment. We will develop this combination as a potential new strategy and use it as an adjunct to infusion chemotherapy in the future.
The authors are grateful for the support provided by the National Natural Science Foundation of China (Grant no. 81172437). The authors appreciate Gansu Nephro- Urological Clinical Center for providing experimental instruments and equipment.
Conflicts of interest
There are no conflicts of interest.
Asghar U, Meyer T (2012). Are there opportunities for chemotherapy in the treatment of hepatocellular cancer? J Hepatol 56:686–695.
Bu XX, Le C, Jia FQ, Guo XL, Zhang L, Zhang BH, et al. (2008). Synergistic effect of mTOR inhibitor rapamycin and fluorouracil in inducing apoptosis and cell senescence in hepatocarcinoma cells. Cancer Biol Ther 7:1–5.
Ca H, Phan H, Yang LX (2012). Improved chemotherapy for hepatocellular carcinoma. Anticancer Res 32:1379–1386.
Chen YC, Tang XJ, Zhang XR, Zhuang LY (2009). New mutations of Nogo-C in hepatocellular carcinoma. Mol Biol Rep 36:377–380.
Danial NN, Korsmeyer SJ (2004). Cell death: critical control points. Cell
Eichhorn ME, Strieth S, Dellian M (2004). Anti-vascular tumor therapy: recent advances, pitfalls and clinical perspectives. Drug Resist Updat 7:125–138. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010). Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer
He W, Wang DM, Ye ZQ, Qian WH, Tao Y, Shi XF, et al. (2012). Application of a nanotechnology antimicrobial spray to prevent lower urinary tract infection: a multicenter urology trial. J Transl Med 10:S14.
Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ (1993). Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 75:241–251. Jemal A, Bray F, Center Melissa M, Ferlay J, Ward E, Forman D (2011). Global
Cancer Statistics. CA Cancer J Clin 61:69–90.
Jia HJ, Li Y, Zhao TS, Li X, Hu JD, Yin D, et al. (2012). Antitumor effects of Stat3- siRNA and endostatin combined therapies, delivered by attenuated
Salmonella, on orthotopically implanted hepatocarcinoma. Cancer Immunol Immunother 61:1977–1987.
Jiao JH, Hong SC, Zhang J, Ma L, Sun Y, Zhang DH, et al. (2012). Opsin3 sensitizes hepatocellular carcinoma cells to 5-fluorouracil treatment by regulating the apoptotic pathway. Cancer Lett 320:96–103.
Johnston S, Martin LA, Leary A, Head J, Dowsett M (2007). Clinical strategies for rational combinations of aromatase inhibitors with novel therapies for breast cancer. J Steroid Biochem Mol Biol 106:180–186.
Lei JC, Yu JQ, Yin Y, Liu YW, Zou GL (2012). Alantolactone induces activation of apoptosis in human hepatoma cells. Food Chem Toxicol 50:3313–3319.
Li W, Ma X, Peng Y, Cao J, Loo WTY, Hao L, et al. (2011). Application of a nano- antimicrobial film to prevent ventilator-associated pneumonia: a pilot study. Afr J Biotechnol 10:1926–1931.
Liu C, Zhang WL, Hu ZL (2010). Clinical study of long-acting antibacterial material Jieyoushen for prevention of incision infection in circumcision. J Chongqing Med Univer 35:1271–1273.
Llovet Josep M (2002). Evidence-based medicine in the treatment of hepa- tocellular carcinoma. J Gastroenterol Hepatol 17:S428–S433.
Lopez PM, Villanueva A, Llovet JM (2006). Systematic review: evidence-based management of hepatocellular carcinoma – an updated analysis of randomized controlled trials. Aliment Pharmacol Ther 23:1535–1547.
Morabito A, Carillio G, Longo R (2009). Systemic treatment of gastric cancer.
Crit Rev Oncol Hematol 70:216–234.
Mukai M, Sato S, Tajima T, Ninomiya H, Wakui K, Komatsu N, et al. (2006). Recurrence and 5-FU sensitivity of stage I/II node-negative breast, lung, or gastric cancer with occult neoplastic cells in lymph node sinuses. Oncol Rep 15:815–820.
Muller C (2006). Hepatocellular carcinoma – rising incidence, changing therapeutic strategies. Wien Med Wochenschr 156:404–409.
Pietra G, Mortarini R, Parmiani G, Anichini A (2001). Phases of apoptosis of melanoma cells, but not of normal melanocytes, differently affect maturation of myeloid dendritic cells. Cancer Res 61:8218–8226.
Ramalingam S, Belani C (2008). Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. Oncologist 13:5–13.
Rocha S, Perkins ND (2005). ARF the integrator-linking NF-kappa B, p53 and checkpoint kinases. Cell Cycle 4:756–759.
Shang DH, Ito N, Watanabe J, Awakura Y, Nishiyama H, Kamoto T, et al. (2007). Synergy of interferon-alpha and 5-fluorouracil in human renal cell carcinoma requires p53 activity. Eur Urol 52:1131–1139.
Siddik ZH (2003). Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 22:7265–7279.
Takayama T, Makuuchi M, Hasegawa K (2010). Single HCC smaller than 2 cm: surgery or ablation?: surgeon’s perspective. J Hepatobiliary Pancreat Sci 17:422–424.
Tanizaki J, Okamoto I, Takezawa K, Tsukioka S, Uchida J, Kiniwa M, et al. (2010). Synergistic antitumor effect of S-1 and HER2-targeting agents in gastric cancer with HER2 amplification. Mol Cancer Ther 9:1198–1207.
Thomas MB, O’Beirne JP, Furuse J, Chan AT, Abou-Alfa G, Johnson P (2008). Systemic therapy for hepatocellular carcinoma: cytotoxic chemotherapy, targeted therapy and immunotherapy. Ann Surg Oncol 15:1008–1014.
Vazquez A, Bond EE, Levine AJ, Bond GL (2008). The genetics of the p53 pathway, apoptosis and cancer therapy. Nat Rev Drug Discov 7:979–987.
Wan XY, Luo M, Li XD, He P (2009). Hepatoprotective and anti-hepatocarcino- genic effects of glycyrrhizin and matrine. Chem Biol Interact 181:15–19.
Wang C, Gao DM, Guo K, Kang XN, Jiang K, Sun C, et al. (2012). Novel synergistic antitumor effects of rapamycin with bortezomib on hepatocellular carcinoma cells and orthotopic tumor model. BMC Cancer 12:166.
Xie Q, Liang BL, Wu YH, Zhang J, Chen MW, Liu HY, et al. (2012). Synergistic anticancer effect of rAd/P53 combined with 5-fluorouracil or iodized oil in the early therapeutic response of human colon cancer in vivo. Gene 499:303–308.
Xu JM, Zhong YS, Niu WX, Qin XY, Lai YH, Ren L, et al. (2007). Preoperative hepatic and regional arterial chemotherapy in the prevention of liver metastasis after colorectal cancer surgery. Ann Surg 245:583–590.
Yang L, Wu DF, Luo KW, Wu SH, Wu P (2008). Andrographolide enhances 5-fluorouracil-induced apoptosis via caspase-8-dependent mitochondrial pathway involving p53 participation in hepatocellular carcinoma (SMMC-7721) cells. Cancer Lett 276:180–188.
Yang XW, Wang XL, Cao LQ, Jiang XP, Peng XF, He P, et al. (2012). Green tea polyphenol epigallocatechin-3-gallate enhances 5-fluorouracil-induced cell growth inhibition of hepatocellular carcinoma cells. Hepatol Res 42:494–501.
Yau T, Chan P, Epstein R (2008). Evolution of systemic therapy of advanced hepatocellular carcinoma. World J Gastroenterol 14:6437–6441.
Yoo BK, Gredler R, Vozhilla N, Su ZZ, Chen D, Forcier T, et al. (2009). Identification of genes conferring resistance to 5-fluorouracil. Proc Natl Acad Sci USA 106:12938–12943.




![]() The synergistic effect of organic silicone quaternary ammonium salt.pdf
The synergistic effect of organic silicone quaternary ammonium salt.pdf
© 2020 南京神奇科技开发有限公司 版权所有 洁悠神学术中心