A Preliminary Report on the Effectivenessof Nanotechnology Anti-Microbial Spray
Dressing in Preventing Tenckhoff
Catheter Exit-Site Infection
For most patients receiving peritoneal dialysis (PD),there is evidence showing that their satisfaction andquality of life have been increasing (1).However, theTenckhoff catheter (TC) can become a potential sourceof infection and peritonitis. If exit-site infection (ESI)is not well managed it can lead to peritonitis or requireremoval of the TC (2).Peritonitis is a well-known causeof mortality in PD patients (3).Consequently, suspend-ing treatment due to access failure may affect patients'overall health status.The purpose of routine care ofthe exit site is to prevent ESI.There is a large volumeof information focused on the prevention of ESI, withdifferent approaches being proposed.The practiceguidelines and protocols from institutions are variedand have not been adequately evaluated, althoughlargevolumes of data have been published on the preventionof ESI(4).
Several recent trial studies show that the applica-tion of JUC Physical Antimicrobial Spray Dressing(NMS Technologies Company Limited,Nanjing,JiangsuProvince,China), has proven to be effective in theprevention of lower urinary tract infection where thespray was applied on the surface of the catheter andthe urethral orifice (5,6), treatment of post-operativeinfection for oral cancer (7), open wound treatment inemergency clinics (8),and managing radiation-inducedacute skin reactions (9). It is also an alternative to anti-biotictreatmenton wound managementfor patients withmethicillin-resistant Staphylococcus aureus(MRSA)infec-tion (10).juC spray dressing was developed in ihna 1n2002 and registered as a dressing product by the United States Food and Drug Administration in 2006.The sprayconsists of 2% organosilicon quaternary ammoniumsalt and 98% distilled water, and is safe for application,even for contact with eyes and mucous memDranes:th scomposed using nano-manufacture technology, yet the antibacterial mechanism is not fully understood. Someproposed mechanisms relate to the physicalstructure ofthe nanoparticles while others relate to the enhancedrelease of antibacterial metal ions from nanoparticlesurfaces which interact with and penetrate into thebacteria (11).
Proper exit-site care is of paramount importance inreducing TC-associated infection and subsequent cath-eter loss.In current practice, patients who have a TCareusually advised to use the traditional antiseptic 0.05% chlorhexidine in exit-site care.Previous studies suggestthat 0.05% chlorhexidine is able to reduce the bacterialload in the wound and promotes cell growth (12).In this study,JUC spray was applied to the TC exit site tocompare the incidence of ESI with the usual standardcare.In addition to ESI,the existence of skin allergy,catheter damage, and time spent on exit-site dressingwere examined.
METHODS
The study was carried out through a randomized con-trolled trial.Patients were recruited from the renalunitof a 1,700-bed acute-care, general regional hospital inHong Kong.Those patients who did not receive oral orexternalantibiotics and who had a TCinplace for at least3 months were recruited sequentially. Patients present-ing with signs and symptoms of exit-site infection andpoor healing of exit site were excluded.There were 121patients assessed for eligibility and 47 subjects wereexcluded.The reasons for exclusion were patients notmeetingthe inclusion criteria or refusingto participate.To compute the sample size, we referred to Li et al.(10)on the effectiveness of JUC spray to prevent ventilator-associated pneumonia.To have 80% power, o.=0.05,todetect a 27.9% reduction in the incidence of bacterialcolonization in the pharyngealcavity in the experimentalgroup compared with the control group, a sample sizeof 35 subjects for each group was required.The catheterwas inserted surgically by open surgical incision.It waswell secured with a dressing and PD was started 4 weeksafter insertion.A 47-cm Argyle Peritoneal curl catheterwith double cuff (Covidien, Mansfield ,MA,02048 USA)was used for both groups of patients.
Atotal of 78 patients were randomized into study orcomparison group by the researcher using computer-generated numbers.Baseline data were collected beforerandomization with masking of treatment allocation.It was a single-blinded study as the patients were notblinded tothe group assignment whilethedata collectorwas blinded to group allocation.The study group patientsusedJUCspraydressing while the comparison group used0.05% chlorhexidine dressing daily for standard woundcare.Coaching was provided by the nurses to ensurethatthe patients were able to per form the procedures cor-rectly.The study team called the patients on the first 3days and they wereinstructedto reportany abnormalitiesthey noticed to the nurses , such as signs and symptoms of infection, skin allergy,and TC damaae.The nafiontswere treated with a full course of antibiotics prescribed by the physician if diagnosed for ESI and they continuedwiththestudy after full recovery fromtreatment.Clinicalefficacy was assessed for a period of 6 months afterimplementation of intervention.
According to the study unit protocol, the presenceof 2 out of 3 equivocal signs and symptoms of ESI wasdiagnosed as acute ESI. Signs and symptomsincluded redcolor around the exit site, with 3 - 4 mm measurementfrom the edge or purulent discharge.
RESULTS
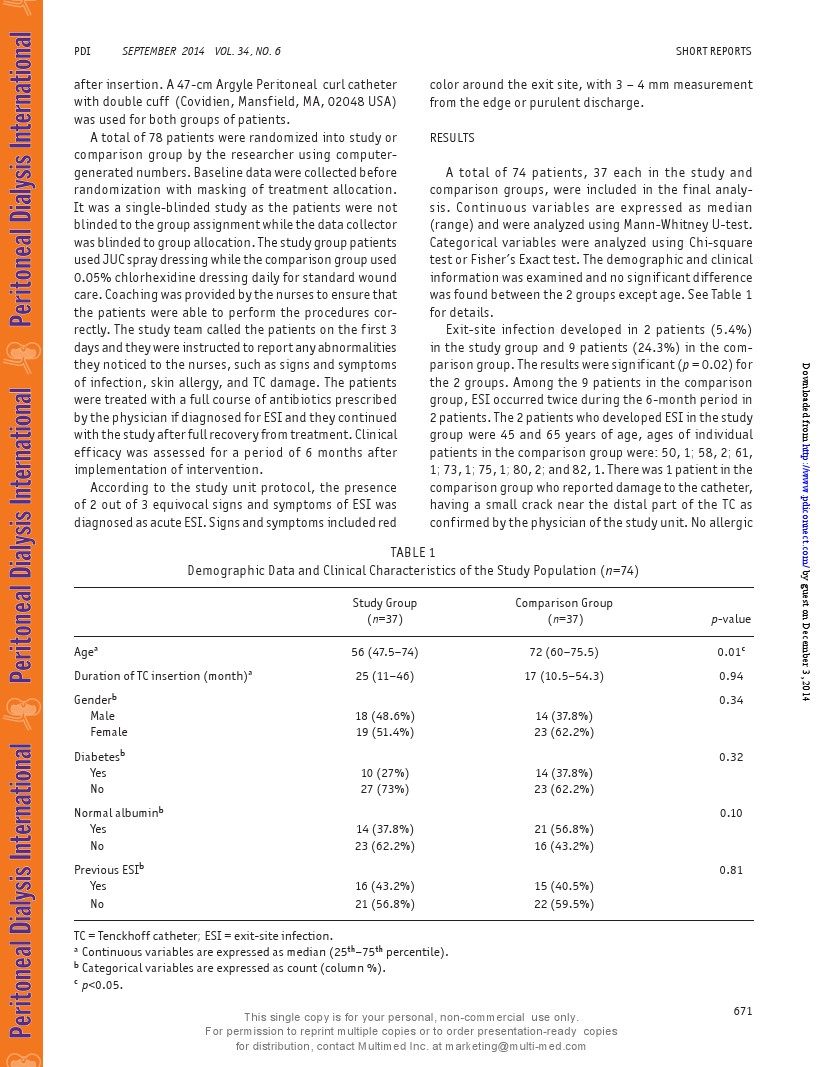
A total of 74 patients, 37 each in the study andcomparison groups,were included in the final analy-sis.Continuous variables are expressed as median(range) and were analyzed using Mann-Whitney U-test.Categorical variables were analyzed using Chi-squaretest or Fisher's Exact test. The demographic and clinicalinformation was examined and no significantdifferencewas found between the 2 groups except age.See Table 1for details.
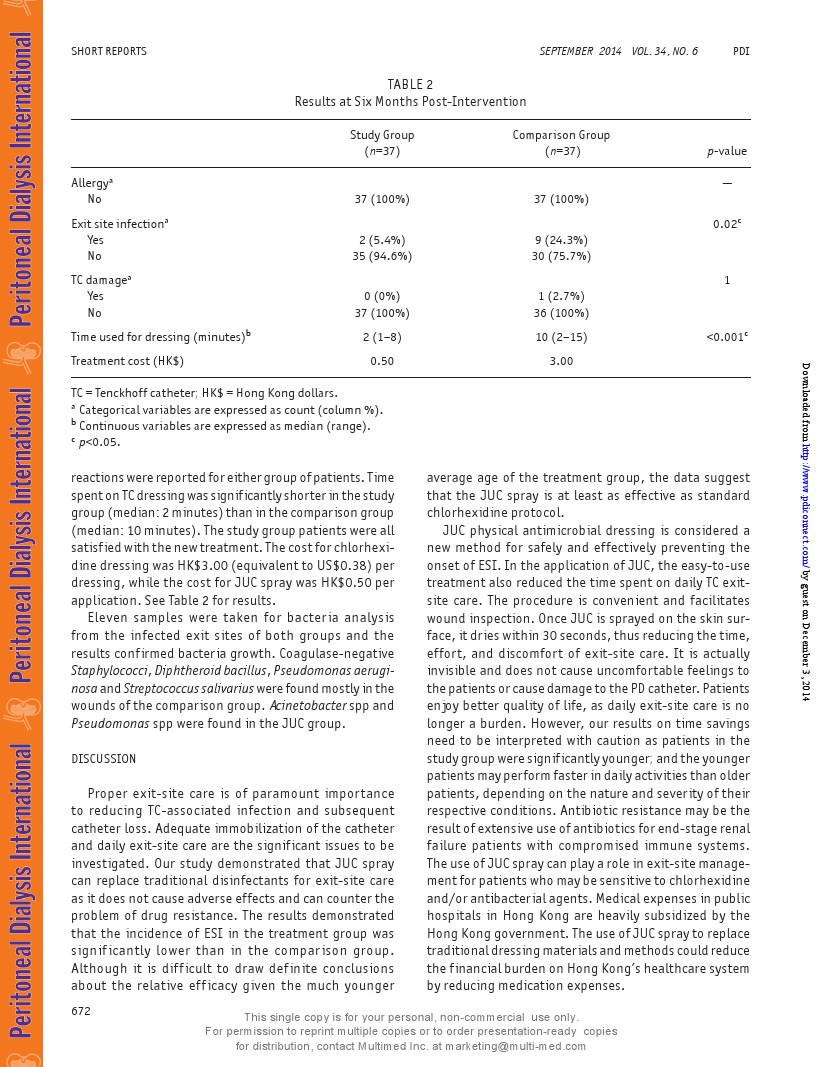
Exit-site infection developed in 2 patients (5.4%)in the study group and 9 patients (24.3%) in the com-parison group. The results were significant(p=0.02) forthe 2 groups.Among the 9 patients in the comparisongroup,ESI occurred twice during the 6-month period in2 patients.The2 patients who developed ESIin the studygroup were 45 and 65 years of age, ages of individualpatients in the comparison group were: 50,1; 58,2; 61,1;73,1; 75,1;80,2; and 82,1.There was 1 patientin thecomparison group who reported damage to the catheter,having a small crack near the distal part of the TC asconfirmed bythe physician of the study unit. No allergicreactions were reported for either group ofpatients.Timespenton TC dressing was significantlyshorterinthe studygroup (median: 2 minutes ) thanin thecomparison group(median: 10 minutes).The study group patients were allsatisfied withthe new treatment.The costfor chlorhexi-dine dressing was HK$3.00(equivalent to US$0.38) perdressing, while the cost for JuC spray was HK$0.50 perapplication. See Table 2 for results.
Eleven samples were taken for bacteria analysisfrom the infected exit sites of both groups and theresults confirmed bacteria growth.Coagulase-negativeStaphylococci, Diphtheroid bacillus, Pseudomonas aerugi-nosaand Streptococcus salivarius were found mostlyin thewounds ofthe comparison group.Acinetobacter spp andPseudomonas spp were found in the JUC group.
DIScUSSION
Proper exit-site care is of paramount importanceto reducing TC-associated infection and subsequentcatheter loss.Adequate immobilization of the catheterand daily exit-site care are the significant issues to beinvestigated.Our study demonstrated that JuC spraycan replace traditional disinfectants for exit-site careas it does not cause adverse effects and can counter theproblem of drug resistance.The results demonstratedthat the incidence of ESI in the treatment group wassignificantly lower than in the comparison group.Although it is difficult to draw definite conclusionsabout the relative efficacy given the much younger average age of the treatment group, the data suggestthat the JuC spray is at least as effective as standardchlorhexidine protocol.
JuC physical antimicrobial dressing is considered anew method for safely and effectively preventing theonset of ESI.In the application of JuC, the easy-to-usetreatment also reduced the time spent on daily TC exit-site care.The procedure is convenient and facilitateswound inspection.OnceJuC is sprayed on the skin sur-face, it dries within 30 seconds, thus reducing the time,effort, and discomfort of exit-site care. It is actuallyinvisible and does not cause uncomfortable feelings tothe patients or cause damage to the PD catheter. Patientsenjoy better quality of life, as daily exit-site care is nolonger a burden. However,our results on time savingsneed to be interpreted with caution as patients in thestudy group were significantly younger; and the youngerpatients may perform fasterin daily activities than olderpatients, depending on the nature and severity of theirrespective conditions.Antibiotic resistance may be theresult of extensive use of antibiotics for end-stage renalfailure patients with compromised immune systems.The use ofJUC spray can play a role in exit-site manage-mentfor patients who may be sensitive to chlorhexidineand/or antibacterialagents. Medical expenses in publichospitals in Hong Kong are heavily subsidized by theHong Kong government.The use ofJUC spray to replacetraditional dressing materials and methods could reducethe financial burden on Hong Kong's healthcare systemby reducing medication expenses.
CONCLUSION
JuC spray is a simple, safe and sustainable techniquefor TC care. Further studies are required using a largersample size to investigate the applicability of JuC inexit-site care for patients from multiple age categoriesresiding in hospital renalunits and in the community.DISCLOSURES
The authors have no financial conflicts of interestto declare.
Bonnie Mee Ling TamSusan Ka Yee Chow*
School of Nursing
The Hong Kong Polytechnic University
Kowloon,Hong Kong
*email: susan.chow@polyu.edu.hk
REFERENCES
1. TokgozB.Clinical advantages of peritonealdialysis.PeritDialInt 2009; 29(Suppl 2):S59-61.
2. Lui SL,Yip KC,Lam MF,Lai KN,Lo WK.Treatment ofrefractory Pseudomonas aeruginosa exit-site infectionby simultaneous removal and reinsertion of peritonealdialysis catheter.Perit Dial Int 2005; 25:560-3.
3. Fontan MP,Rodriguez-Carmona A, Garcia-Naveiro R,Rosales M,Villaverde P, Valdes F. Peritonitis-related mortality in patients undergoing chronic peritonealdialysis.Perit Dial Int 2005;25:274-84.
4. Bender FH,Bernardini J,Piraino B.Prevention of infec-
tious complications in peritoneal dialysis: best demon-strated practices.Int Soc Nephrol2006; 70:S44-54.5. Wu L, DaiYT, Wang LM, Cheng B, Sun ZY.Study on preven-
tion of catheterassociated urinarytractinfection by usingJUS long-acting antibacterial material. Nation J Andro2005; 11:581-3.
6. He W, Wang D, YeZ,Qian W,Tao Y,ShiX, et al.Application
of a nanotechnology antimicrobialspray to preventlowerurinary tract infection: a multicenterurology trial.J TransMed 2012; 10(Suppl 1):S14.
7. Zeng Y,Deng R, Yeung B,Loo W, Cheung M, Chen JP, et al.Application of an antibacterial dressing spray in the pre-vention ofpost-operative infectionin oralcancer patients:a phase 1 clinical trial.AfrJ Biotechnol 2008; 7:3827-31.8. Shen M,Li7.JUC long-acting antimicrobial material in adjuvant treatment of 129 cases of open wound.HeraldMed,2006; 25(2):138-9.
9. Wan K,Ng MY,Wong YT. New horizon on community-acquired methicillin-resistant Staphylococcus aureus(CA-MRSA) skinand softtissueinfection: nanotechnologyantimicrobial spray.HK J Emerg Med 2011; 18:432-6.10. Li W,Ma X,Peng Y,Cao J,Loo TY,Hao L, et al.Applica-tion of a nano-antimicrobial film to prevent ventilator-associated pneumonia: a pilotstudy.Afr JBiotechnol 2011;10:1926-31.
11. Seil JT,Webster TJ.Antimicrobial applications of nano-technology: methods and literature.Int J Nano 2012;7:2767-81.
12. Drosou A, Falabella A, Kirsner RS.Antiseptics on wounds:an area of controversy. Wounds 2003; 15:149-66.


![]() A preliminary report on the effectiveness of Nanotechnology Anti-Microbial Spray.pdf
A preliminary report on the effectiveness of Nanotechnology Anti-Microbial Spray.pdf
© 2020 南京神奇科技开发有限公司 版权所有 洁悠神学术中心