Application of an antibacterial dressing spray in the prevention of post-operative infection in oral cancer patients: A phase 1 clinical trial
Yizhou Zeng1, Runzhi Deng1*, Barry, H.S. Yeung2, Wings T.Y. Loo3, Mary N.B. Cheung3, J.P. Chen3, Bingrong Zhou1, Yifu Fu1, Lanzhu Huang1, Mingxing Lu1 and Min Wang4
1Department of Oral Maxillofacial Surgery, Nanjing Stomatological Hospital, Nanjing, China.
2Department of Microbiology, Immunology and Molecular Genetics, the University of California Los Angeles, USA.
3School of Chinese Medicine, the University of Hong Kong, Hong Kong.
4Department of Prosthodontics, West China College of Stomatology, University of Sichuan, Chengdu, P.R. China.
Accepted 8 October, 2008
Key words:Antibacterial dressing spray, oral squamous cell cancinoma, wound healing, infection.
Oral cavity interlinks with respiratory tracts and digestive tracts. After surgery in these tracts, aerobic and anaero- bic bacteria frequently induce operative wound infections in teeth, periodontal and supporting tissues of the teeth and tonsils (Senpuku et al., 2002, 2006; Salam et al., 2001). It is found that these infected areas generally have beneficial environment for bacterial proliferation; suitable temperature and humidity, which account for the frequent infections.
In general, infections are commonly found in oral can- cer patients after surgical excision of the tumor (Senpuku et al., 2003, 2006; Tada, 2002a, b). This might be due to wound exposure during and after the operation, even if sutured, which micro-organisms may infect oral regions,oropharynx, nasal cavity, and paranasal sinuses areas. Patients after oral cavity surgery often appear to have complications after bacterial infections, as well. Coloniza- tion of pathogenic bacteria in oral cavity is thought to increase the risk of infections such as pneumonia and bacteraemia (Costerton et al., 1999; Gosney et al, 1999). It is therefore of high importance to prevent from or cure for the infections.
Currently, the systemic applications of antibacterial drugs have shown better results on curing diseases than local application, which could induce drug-resistant bacteria in the particular area (Belusic-Gobic et al., 2007; Cloke et al., 2004). In this study, however, JUC physical antimicrobial dressing was applied to some specific areas on oral cancer patients after surgery to prove the new physical antimicrobial method, which will not lead to the production of drug resistant bacteria, while preventing infections.

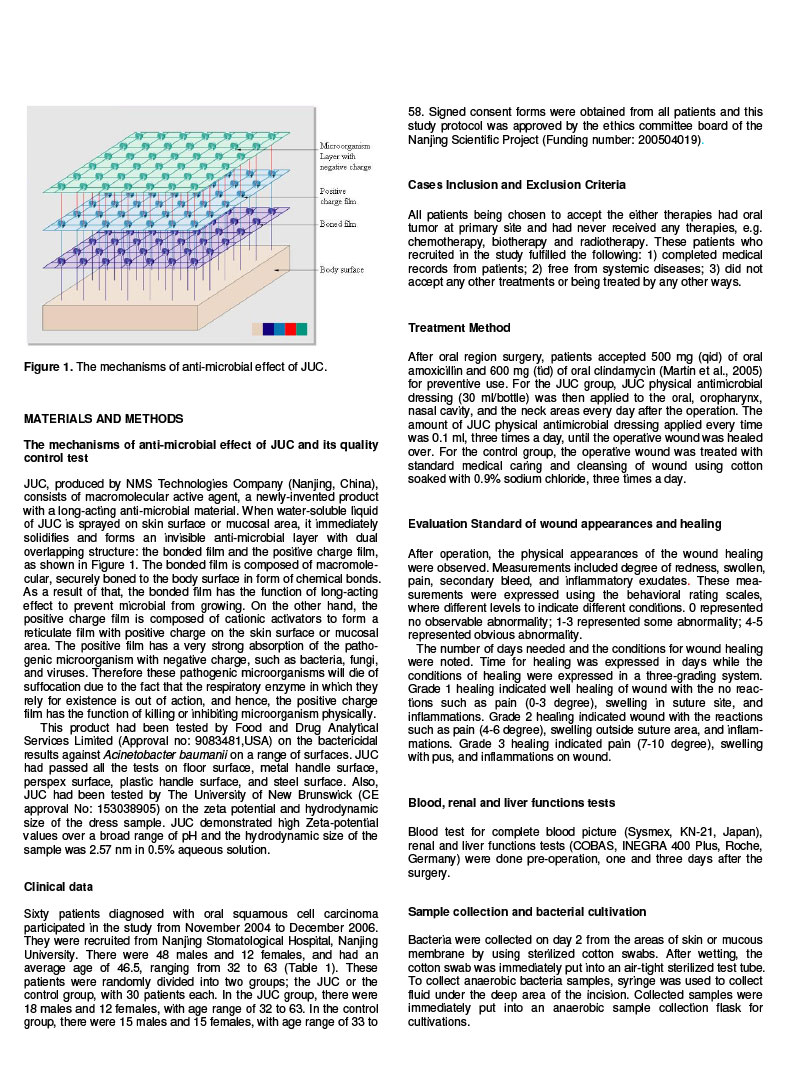
Figure 1. The mechanisms of anti-microbial effect of JUC.
MATERIALS AND METHODS
The mechanisms of anti-microbial effect of JUC and its quality control test
JUC, produced by NMS Technologies Company (Nanjing, China), consists of macromolecular active agent, a newly-invented product with a long-acting anti-microbial material. When water-soluble liquid of JUC is sprayed on skin surface or mucosal area, it immediately solidifies and forms an invisible anti-microbial layer with dual overlapping structure: the bonded film and the positive charge film, as shown in Figure 1. The bonded film is composed of macromole- cular, securely boned to the body surface in form of chemical bonds. As a result of that, the bonded film has the function of long-acting effect to prevent microbial from growing. On the other hand, the positive charge film is composed of cationic activators to form a reticulate film with positive charge on the skin surface or mucosal area. The positive film has a very strong absorption of the patho- genic microorganism with negative charge, such as bacteria, fungi, and viruses. Therefore these pathogenic microorganisms will die of suffocation due to the fact that the respiratory enzyme in which they rely for existence is out of action, and hence, the positive charge film has the function of killing or inhibiting microorganism physically.
This product had been tested by Food and Drug Analytical Services Limited (Approval no: 9083481,USA) on the bactericidal results against Acinetobacter baumanii on a range of surfaces. JUC had passed all the tests on floor surface, metal handle surface, perspex surface, plastic handle surface, and steel surface. Also, JUC had been tested by The University of New Brunswick (CE approval No: 153038905) on the zeta potential and hydrodynamic size of the dress sample. JUC demonstrated high Zeta-potential values over a broad range of pH and the hydrodynamic size of the sample was 2.57 nm in 0.5% aqueous solution.
Clinical data
Sixty patients diagnosed with oral squamous cell carcinoma participated in the study from November 2004 to December 2006. They were recruited from Nanjing Stomatological Hospital, Nanjing University. There were 48 males and 12 females, and had an average age of 46.5, ranging from 32 to 63 (Table 1). These patients were randomly divided into two groups; the JUC or the control group, with 30 patients each. In the JUC group, there were 18 males and 12 females, with age range of 32 to 63. In the control group, there were 15 males and 15 females, with age range of 33 to58. Signed consent forms were obtained from all patients and this study protocol was approved by the ethics committee board of the Nanjing Scientific Project (Funding number: 200504019).
Cases Inclusion and Exclusion Criteria
All patients being chosen to accept the either therapies had oral tumor at primary site and had never received any therapies, e.g. chemotherapy, biotherapy and radiotherapy. These patients who recruited in the study fulfilled the following: 1) completed medical records from patients; 2) free from systemic diseases; 3) did not accept any other treatments or being treated by any other ways.
Treatment Method
After oral region surgery, patients accepted 500 mg (qid) of oral amoxicillin and 600 mg (tid) of oral clindamycin (Martin et al., 2005) for preventive use. For the JUC group, JUC physical antimicrobial dressing (30 ml/bottle) was then applied to the oral, oropharynx, nasal cavity, and the neck areas every day after the operation. The amount of JUC physical antimicrobial dressing applied every time was 0.1 ml, three times a day, until the operative wound was healed over. For the control group, the operative wound was treated with standard medical caring and cleansing of wound using cotton soaked with 0.9% sodium chloride, three times a day.
Evaluation Standard of wound appearances and healing
After operation, the physical appearances of the wound healing were observed. Measurements included degree of redness, swollen, pain, secondary bleed, and inflammatory exudates. These mea- surements were expressed using the behavioral rating scales, where different levels to indicate different conditions. 0 represented no observable abnormality; 1-3 represented some abnormality; 4-5 represented obvious abnormality.
The number of days needed and the conditions for wound healing were noted. Time for healing was expressed in days while the conditions of healing were expressed in a three-grading system. Grade 1 healing indicated well healing of wound with the no reac- tions such as pain (0-3 degree), swelling in suture site, and inflammations. Grade 2 healing indicated wound with the reactions such as pain (4-6 degree), swelling outside suture area, and inflam- mations. Grade 3 healing indicated pain (7-10 degree), swelling with pus, and inflammations on wound.
Blood, renal and liver functions tests
Blood test for complete blood picture (Sysmex, KN-21, Japan), renal and liver functions tests (COBAS, INEGRA 400 Plus, Roche, Germany) were done pre-operation, one and three days after the surgery.
Sample collection and bacterial cultivation
Bacteria were collected on day 2 from the areas of skin or mucous membrane by using sterilized cotton swabs. After wetting, the cotton swab was immediately put into an air-tight sterilized test tube. To collect anaerobic bacteria samples, syringe was used to collect fluid under the deep area of the incision. Collected samples were immediately put into an anaerobic sample collection flask for cultivations.
Table 1. Characteristics of subjects.
Parameters | Patients Number and references values (n=60) | |
Age Sex Tumor position Tumour type T1-T2 N0,M0 | 46.5 (range: 32-63) 48 Male12 Female 48% in tongue52% in floor of mouth Squamous cell carcinoma 60 | |
Grade 1 surgery excises | 2 mm | 60 |
T, tumour; N, nodes; and M, metastases.
Table 2. Grades of healing after tumor excision by using JUC or traditional methods.
Grade of Healing | JUC group (n=30) | Control Group (n=30) |
1 | 26* | 22 |
2 | 4* | 8 |
3 | 0 | 0 |
*Indicates statistical significant (p<0.05) between two group.
Table 3. Wound healing time after tumor excision by using JUC or traditional methods.
JUC group (n=30) | Control Group (n=30) | |
Average Wound Healing Time | *8.97 ± 1.22 days | 9.74 ±1.32 days |
*Indicates statistical significant (p<0.05) between two group.
Statistical analysis
Statistical analyses were done using SPSS 15.0 (SPSS Inc., U.S.A.) and any differences at p<0.05 level were considered as statistically significant. The grading evaluation of symptoms and healing for control and patient groups were analyzed with chi-squared test to access statistical significance. All parameters of samples of both groups were compared using independent student t-test.
Physical appearance of wounds
Among all 60 patients from both groups, all received conservation surgery of the oral cavity. Among these cancer patients, their physical signs showed that values were within normal range after excision. Swelling of wound was reduced three to five days after surgery in JUC group. Their body temperature range was 37.8-38.8 with mild fever for the first 2 days after surgery.
For all patients, none of them had grade 3 healing. Thenumbers of grade 1 and grade 2 healing patients pre- sented in the JUC group were more than that in the control group with significant differences (p<0.05) (Table 2).
The healing time for patients who applied JUC was shorter than that of patients in the control group, who received traditional methods. The JUC group had wound healed in 8.97 days while it required 9.74 days for wound healing in the control group (Table 3).
For patients who received JUC physical antimicrobial dressing and patients who had received traditional oral administrative drug, all of them did not present with any side effects.
The blood, kidney and liver functions assay, did not show any significant differences between and after treatment for both groups which received JUC physical antimicro- bial dressing or traditional oral care methods (p>0.05) (Tables 4 and 5)
No obvious bacteria was cultured from skin or mucosal areas. Fifty samples suctioned from the incision beneath showed bacteria growth. Streptococcus, Staphylococcus, Veillonella, Neisseria, and Actinomyces were found mostly in the wound. For Streptococcus, Staphylococcus, and Neisseria there were significant differences (p<0.05) in amount between both groups (Table 6). In JUC group, the amount of Streptococcus, Staphylococcus, and Neisseria were significantly lower than that of the control group using traditional oral care methods. For Veillonella and Actinomyces, no significant differences were shown between the two groups (Table 6).
Table 4. Blood picture of white and red cells for both groups receiving JUC or traditional oral-caring methods (control) on days 0, 1 and 3.
Parameter |
Unit | Normal Range | Day 0 | Day 1 | Day 3 | |||
Control | JUC | Control | JUC | Control | JUC | |||
White blood cell Granulocytes Lymphocytes Hemoglobin Platelet | x109/L x109/L x109/L g/L x109/L | 4.00-11.00 0.2-1.30 1.50-4.00 115-165 150-400 | 6.2 ± 2.5 0.4 ± 0.33 1.25 ± 0.89 140 ± 22 142 ± 35 | 5.7 ± 2.8 0.4 ± 0.46 1.0 ± 1.3 138 ± 34 122 ± 42 | 10.8 ± 1.57 0.8 ± 0.26 1.85 ± 2.0 140 ± 27 135 ± 41 | 11.9 ± 1.8 0.9 ± 0.18 1.84 ± 2.2 168 ± 28.9 146 ± 50 | 7.4 ± 3.2 0.6 ± 0.32 1.86 ± 1.13 135 ± 36 125 ± 48 | 7.8 ± 4.0 0.64 ± 0.41 1.86 ± 1.3 190 ± 33 170 ± 152 |
Data are presented as mean ± 95% confidence interval (95% CI).
Table 5. Renal and Kidney functions tests for both groups received JUC or traditional oral-caring (control) methods on days 0, 1 and 3.
Parameter | Unit | Normal Range | Day 0 | Day 1 | Day 3 | |||
JUC | Control | JUC | Control | JUC | Control | |||
SGOT/ALT | U/L | 5-31 | 30 ± 0.5 | 28 ± 0.23 | 30 ± 7.0 | 29.5 ± 7.8 | 28 ± 10.5 | 26 ± 3.5 |
SGPT/ALT | U/L | 12-28 | 24 ± 1.0 | 25 ± 0.98 | 26 ± 0.45 | 28 ± 0.76 | 27 ± 0.45 | 24 ± 1.36 |
Albumin | g/L | 42-54 | 40 ± 3.0 | 41 ± 3.2 | 38 ± 4.4 | 40 ± 5.4 | 37 ± 6.4 | 40 ± 5.8 |
GLB | g/L | 20-30 | 24 ± 3.0 | 23 ± 3.5 | 22 ± 4.6 | 26 ± 5.0 | 20 ± 6.8 | 22 ± 7.6 |
Bilirubin | umol/L | 7-19 | 5.6 ± 1.8 | 6.1 ± 1.48 | 8.7 ± 2.4 | 6.5 ± 2.8 | 5.6 ± 3.8 | 4.9 ± 3.5 |
Creatinine | umol/L | 44-106 | 96 ± 11 | 97 ± 10 | 86 ± 13 | 96 ± 9.5 | 94 ± 8.8 | 84 ± 9.6 |
Data are presented as mean ± 95% confidence interval (95% CI).
Table 6. Bacterial growth inside incision after surgical excision.
Bacteria | JUC Group (n=28) | Control Group (n=22) | ||
No. of positive cases | Percentage | No. of positive cases | Percentage | |
Streptococcus | 8 | 28.6% | 13 | 59.1%* |
Staphylococcus | 9 | 32.1% | 14 | 63.6%* |
Veillonella | 10 | 35.7% | 10 | 45.5% |
Neisseria | 7 | 25.0% | 12 | 54.5%* |
Actinomyces | 9 | 32.1% | 12 | 54.5% |
*There were significant differences between the two groups (p<0.05).
Patients with tumors are easily affected with bacterial infections. They may have poorer immunity, malnutrition, anemia, and they appear to be weaker than before. Dur- ing the development of tumor, the tumor cells or soluble products produced by tumor cells inactivate lymphocytes and provoke immunosuppression in the body (Bennett et al., 1996; Camp et al., 1996; Gimmi et al, 1996; Jewett et al., 2006; Mulder et al, 1996). Nevertheless, anti-tumor drugs used in tumor treatment will also lead to immune and bone marrow suppression. Therefore, infections can therefore occur at both operated (Senpuku et al., 2003, 2006; Tada et al., 2002a, b) and non-operated sites (Angele and Faist, 2002; Götzinger et al., 1996; Tedder et al., 1994; Yehia et al., 2004) and any small cuts in daily lives can bring tumor patients life-threaten infections.A long operation time in oral cavity raises the chances of bacterial infections (Angele and Faist, 2002; Götzinger et al., 1996). Therefore, effective preventive measures are necessary to protect the patients.
Nowadays, antimicrobial drugs have been very helpful to prevent infections after surgery. People, however, sometimes misuse them and have antimicrobial drugs abuse. The abuse of antimicrobial drugs brings severe adverse effects to people, for example, allergy, toxic reaction, and opportunistic infections (Senpuku et al., 2006). As a result, a new and ideal preventive method is needed for people who have surgery to reduce the chances of bacterial infections.
JUC physical antimicrobial dressing is a newly- invented treatment for patients who have had surgery (NMS Technologies Company Nanjing, China). It is made up of organosilicon quaternary ammonium salt, and a water-soluble liquid of macromolecular active spraying agent. These form a positive film, attracting the nega- tively charged microorganisms. These microorganisms will die and are impossible to exchange materials, which lead to physical antimicrobial purpose. The JUC physical antimicrobial dressing is a good method which will not lead to the production of drug resistant bacteria, and it also gives a new way to help prevent bacterial infections.
The result of the experiment revealed that using the JUC physical antimicrobial dressing caused no serious adverse stimulations to the patients. All patients had good healing progress with no grade 3 healings but while using JUC physical antimicrobial dressing, the number of grade 1 and grade 2 wound healing increased and the average healing time required was shortened (p<0.05), which was significantly shorter than the group using traditional oral caring methods. For the analysis of blood parameters, kidney and liver functions, there were no significant differences between the groups using JUC or traditional oral caring methods (p>0.05). This indicated that application of JUC did not cause any significant side effects to the body; being the same as using traditional oral caring methods. On the other hand, JUC had a better inhibition effect on the growth of anaerobes than traditional oral caring methods (p<0.05) in which these anaerobic bacteria grew under the incision were those which possibly cause infections to the wound.
During application of JUC physical antimicrobial dressing, special care should be made since oral and maxillofacial are very complex structures with lots of saliva secretions. It is suggested that before application of JUC, spraying of dressing should be evenly distributed onto the whole surface of the incision right after cleaning of the incision such that the positive film can be formed and should cover the whole surface of the incision, hence, facilitating the healing of wounds. If sutures have to be taken out after healing, it can be done simply by removing the thin film of dressing and re-spraying JUC onto the surface of the incision after the removal of suture.
In conclusion, application of JUC physical antimicrobial dressing had beneficial effects over traditional oral caring methods. JUC shortened the healing of wounds by inhibiting the growth of some anaerobic bacteria without bringing any significant side effects. This easy-to-use treatment to prevent bacterial infection could be applied to patients after oral and maxillofacial tumor removal surgery.
REFERNCES
Angele MK, Faist E (2002). Clinical review: immunodepression in the surgical patient and increased susceptibility to infection. Crit Care, 6: 298-305.
Belusic-Gobic M, Car M, Juretic M, Cerovic R, Gobic D, Golubovic V (2007). Risk factors for wound infection after oral cancer surgery. Oral Oncol. 43(1): 77-81.
Bennett MW, O’Connell J, O’Sullivan GC, Brady C, Roche D, Collins JK et al Provide names of other authors (1996). The Fas counterattack in vivo: apoptotic depletion of tumor-infiltrating lymphocytes associa-=with Fas ligand expression by human esophageal carcinoma. J. Immunol. 160: 5669-5675.
Camp BJ, Dyhrman ST, Memoli VA, Mott LA, Barth RJ (1996). In situ cytokine production by breast cancer tumor-infiltrating lymphocytes. Ann. Surg. Oncol. 3: 176-184.
Cloke DJ, Green JE, Khan AL, Hodgkinson PD (2004). McLean NR. Factors influencing the development of wound infection following free-flap reconstruction for intra-oral cancer. Br. J. Plast. Surg. 57: 556-60.
Costerton JW, Stewart PS, Greenberg EP (1999). Bacterial biofilms: a common cause of persistent infections. Science, 284: 1318-22.
Gimmi CD, Morrison BW, Mainprice BA, Gribben JG, Boussiotis VA, Freeman GJ et al Provide names of other authors (1996). Breast cancer-associated antigen, DF3/MUC1, induces apoptosis of activated human T cells. Nat Med. 2: 1367-1370.
Gosney MA, Preston AJ, Corkhill J, Millns B, Martin MV (1999). Pseudomonas aeruginosa septicaemia from an oral source. Br. Dent. J. 187: 639-40.
Götzinger P, Sautner T, Wamser P, Gebhard B, Barlan M, Steininger R, Függer R, Mühlbacher F (1996). Early postoperative infections after liver transplantation--pathogen spectrum and risk factor. Wien Klin Wochenschr. 108: 795-801.
Jewett A, Head C, Cacalano NA (2006). Emerging mechanisms of immunosuppression in oral cancers. J. Dent. Res. 85(12): 1061- 1073.
Martin MV, Kanatas AN, Hardy P (2005). Antibiotic prophylaxis and third molar surgery. BDJ. 198: 327-330.
Mulder WM, Bloemena E, Stukart MJ, Kummer JA, Wagstaff J, Scheper RJ (1996). T cell receptor-zeta and granzyme B expression in mononuclear cell infiltrates in normal colon mucosa and colon carcinoma. Gut. 40: 113-119.
Salam MA, Senpuku H, Nomura Y, Matin K, Miyazaki H, Hanada Nm (2001). Isolation of opportunistic pathogens in dental plaque, saliva and tonsil samples from elderly. Jpn. J. Infect. Dis. 54: 193-195.
Senpuku H, Sogame A, Inoshita E, Tsuha Y, Miyazaki H, Hanada N (2003). Systemic disease in association with microbial species in oral biofilm from elderly requiring care. Gerontology, 49: 301-309.
Senpuku H, Tada A, Takada M, Sato Y, Hanada N (2002). Repro- ducibility of oral bacterial isolation in the elderly. Jpn. J. Infect. Dis. 33: 61-62.
Senpuku H, Tada A, Uehara S, Kariyama R, Kumon H (2006). Post- operative infection by pathogenic micro-organisms in the oral cavity of patients with prostatic carcinoma. J. Int. Med. Res. 34(1): 95-102.
Tada A, Hanada N, Tanzawa H (2002). The relation between tube feeding and pseudomonas aeruginosa detection in the oral cavity. J. Gerontol. A Biol. Sci. Med. Sci. 57: M71-72.
Tada A, Watanbe T, Yokoe H, Hanada N, Tanzawa H (2002b). Oral bacteria influenced by the functions status of the elderly people and type and quality of facilities for the bedridden. J. Appl. Microbiol. 93: 487-491.
Tedder M, Spratt JA, Anstadt MP, Hegde SS, Tedder SD, Lowe JE (1994). Pulmonary mucormycosis: results of medical and surgical therapy. Ann. Thorac. Surg. 57: 1044-1050.
Yehia M, Thomas M, Pilmore H, Van Der Merwe W, Dittmer I (2004). Subcutaneous black fungus (phaeohyphomycosis) infection in renal transplant recipients: three cases. Transplantation, 77: 140-142


![]() Application of an antibacterial dressing spray .pdf
Application of an antibacterial dressing spray .pdf
© 2020 南京神奇科技开发有限公司 版权所有 洁悠神学术中心